Introduction
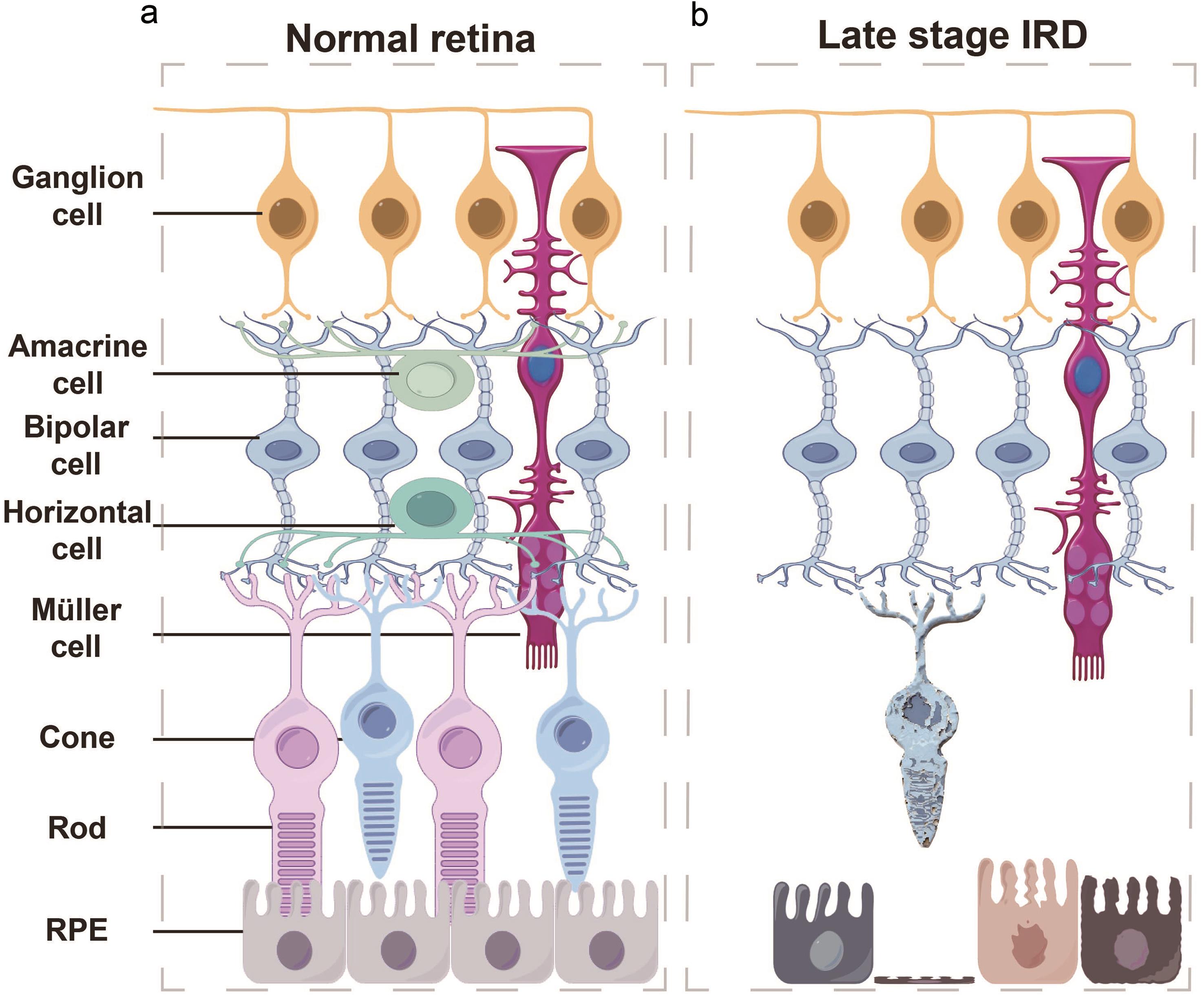
Retinal degenerative diseases (RDDs) involve the gradual deterioration of vision due to the progressive loss of retinal neurons, photoreceptors, and the underlying retinal pigment epithelium (RPE) (Fig. 1). Given the limited regenerative potential of the adult mammalian retina, the death of these cells often leads to irreversible visual deficits. Prominent RDDs include age-related macular degeneration (AMD),1 retinitis pigmentosa (RP),2 and Stargardt’s disease.3 To develop effective therapeutic approaches, significant efforts have been devoted to elucidating the genetic and molecular mechanisms underlying these pathological processes. These efforts highlight that the progression of these diseases results from complex interactions among genetic, epigenetic, metabolic, and microenvironmental factors.
(a) Conceptual diagram of cell types and their distribution in the normal human retina. (b) Schematic representation of end-stage inherited retinal degeneration, depicting complete photoreceptor loss with relative preservation of the inner retinal layers. IRD, Inherited retinal diseases; RPE, retinal pigment epithelium.
Genetic mutations and epigenetic changes jointly disrupt retinal homeostasis, leading to progressive visual impairment. To date, more than 330 disease-causing genes have been identified in multiple RDD phenotypes (RETNET; https://retnet.org , accessed on June 28, 2025). The proper function of retinal cells relies on genes such as UBAP1L, ALG6, and IDH3G.4–6 In addition to monogenic mutations, polygenic influences play a substantial role, with the cumulative effect of multiple risk alleles modulating disease onset and progression.7–9 Epigenetic modifications, including DNA methylation, histone modifications, and non-coding RNA regulation, add another layer of complexity.10 These heritable yet reversible changes in gene expression offer deeper insights into the dynamic epigenetic landscape of the retina. Furthermore, genetic predispositions may combine with environmental stressors such as smoking, ultraviolet radiation, and air pollution-induced oxidative stress to increase the risk of disease development.11
Oxidative stress and mitochondrial dysfunction are central to retinal degeneration.12,13 The high metabolic activity of the retina makes it susceptible to the accumulation of reactive oxygen species (ROS), which can trigger cellular damage like mitochondrial dysfunction. Mitochondrial dysfunction exacerbates ROS production, creating a feedback loop that accelerates photoreceptor cell death.13 Inflammation and immune dysregulation also play pivotal roles, with microglial activation, peripheral immune cell infiltration, and cytokine network dysregulation disrupting retinal homeostasis.14 Additionally, protein misfolding and endoplasmic reticulum (ER) stress activate the unfolded protein response (UPR), which can turn from protective to pro-apoptotic under chronic stress.15–17 Impaired autophagy and ubiquitination further contribute to the accumulation of misfolded proteins and cellular stress.18,19 Vascular abnormalities and extracellular matrix (ECM) changes are also key factors, with studies showing significant microvascular degeneration and ECM protein expression changes in RDDs.20,21 Overall, these molecular mechanisms highlight the multifactorial nature of RDDs and provide potential targets for therapeutic intervention.
In recent years, significant progress has been made in cell-based therapies, pharmacological interventions, gene therapy, and regenerative medicine. Pharmacological interventions play a crucial role in managing retinal degeneration by targeting the underlying molecular mechanisms. Major developments include the evolution of anti-vascular endothelial growth factor (anti-VEGF) agents, the investigation of repurposed drugs with neuroprotective potential, and the optimization of targeted intraocular drug delivery systems.22–24 Advances in gene therapy vectors and delivery methods have opened new possibilities for treating and potentially curing RDDs.25 Furthermore, advances in stem cell technologies and bioengineering have brought cellular therapies closer to clinical application,26–29 although challenges such as immune compatibility and functional integration still persist.30,31 Overall, these therapeutic approaches offer promising avenues for addressing previously untreatable retinal disorders.
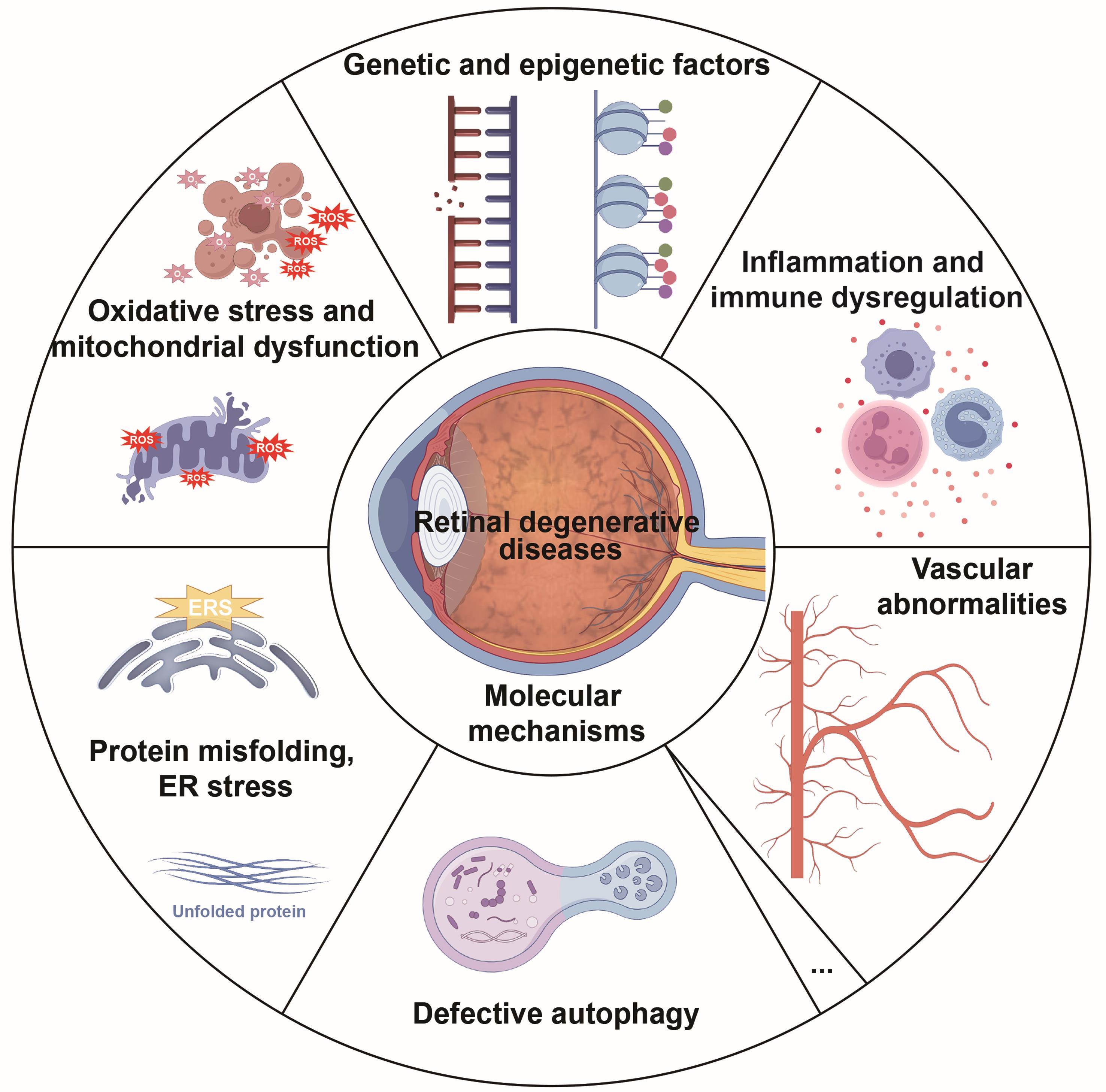
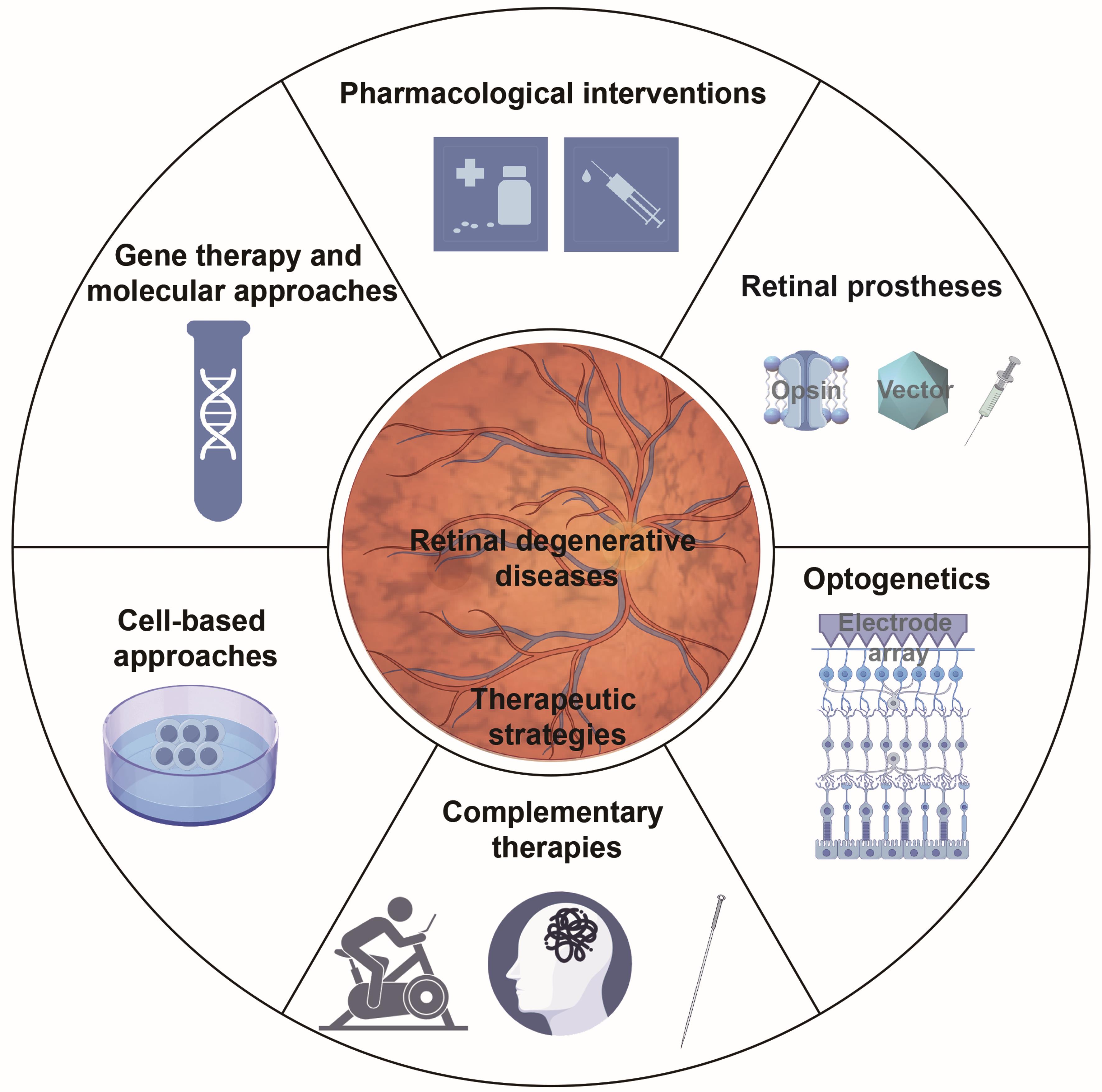
A central objective of this review is to scrutinize the latest fundamental investigations and clinical trials related to retinal degeneration published in recent years, reflecting the field’s rapid progress. We investigated the molecular mechanisms underlying retinal degeneration (Fig. 2), assessed current and emerging treatment approaches (Fig. 3), and analyzed the obstacles to translating these therapies from the laboratory to clinical practice. This review aims to provide a comprehensive and clear overview of the current status of RDDs.
The diagram summarizes the principal molecular pathways implicated RDDs, including genetic and epigenetic alterations, oxidative stress and mitochondrial dysfunction, dysregulated inflammation and immunity, protein misfolding with endoplasmic reticulum (ER) stress and autophagy imbalance, and vascular and extracellular matrix abnormalities. ERS, endoplasmic reticulum stress; ROS, reactive oxygen species.
The diagram depicts the primary treatment modalities for RDDs, including pharmacological treatments, gene- and molecular-based strategies, cell therapy approaches, retinal prosthetic devices, optogenetic interventions, and complementary therapeutic measures.
Molecular mechanisms
Genetic and epigenetic factors
RDDs, including AMD and RP, are multifactorial disorders in which genetic mutations and epigenetic dysregulation jointly disrupt retinal homeostasis and drive progressive visual impairment.32,33 Extensive genetic studies have pinpointed key genes associated with these conditions. For instance, Ullah et al.6 demonstrated that biallelic loss-of-function variants in UBAP1L are implicated in human nonsyndromic retinal dystrophies. Monson et al.5 reported that the ALG6 variant is correlated with increased severity of macular cone dysfunction but milder peripheral rod involvement. Bianco et al.4 reported that IDH3G, which encodes the γ-subunit of mitochondrial isocitrate dehydrogenase and is expressed in photoreceptor inner segments, is a new candidate for X-linked RP. In addition to these monogenic mutations, polygenic influences play a substantial role in retinal degeneration. The cumulative impact of multiple risk alleles can modulate both the onset and progression of the disease, emphasizing the importance of rare and common variants alike.7–9 Gorman et al.7 combined data from the Million Veteran Program and five other cohorts to conduct pioneering multi-ancestry AMD genome-wide association studies, discovering 63 loci in total, 30 of which were novel.
Epigenetic modifications are equally significant in the pathogenic process. These are heritable yet reversible changes in gene expression that occur without altering the underlying DNA sequence. Such modifications include DNA methylation, histone modifications, and regulation by noncoding RNAs. Wahlin et al.34 initially reported irregular DNA methylation levels in the rd1 mouse model of RP. At timepoints corresponding to the peak of rod cell death, both rod and cone photoreceptors in the rd1 retina displayed heightened immunoreactivity for 5mC and 5hmC relative to wild-type controls. Recent progress in high-throughput sequencing and epigenomic profiling has provided a more profound understanding of the dynamic epigenetic landscape of the retina. Advani et al.35 carried out integrated RNA sequencing and DNA methylation array analyses on 160 human retinal samples, identifying 37,453 methylation QTLs and 13,747 DNA methylation sites that influence gene expression. Summary data-based Mendelian randomization and colocalization analyses revealed 87 target genes whose methylation and gene expression changes likely affect the genotype of AMD. Histone modifications present an extra regulatory tier by adjusting chromatin structure and swaying transcription factor access to gene promoters. For example, dysregulation of EZH2 (a catalytic component of polycomb repressive complex 2) activity is correlated with abnormal cell cycle progression and apoptosis in retinal cells. Moreover, changes in histone acetylation patterns are connected to the repression of neuroprotective genes and the aggravation of inflammatory pathways.36 Moreover, the homeostasis of non-coding RNAs is vital for the maturation and survival of different retinal cells, as well as for maintaining the normal structure and function of the retina. For example, miR-20b restrains photoreceptor cell proliferation and development and promotes apoptosis by targeting fibroblast growth factor 2 and growth factor receptor-bound protein 2 through the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway.37 MiR-210 is essential for retinal cell integrity and survival, and its absence triggers retinal degeneration.38 MiR-210 also regulates lipid metabolism by targeting acetyl-CoA synthase, thereby preventing neurodegeneration in the Drosophila retina.39 Let-7a/miR-125b,40 miR-20b/106a, and miR-204/211 play roles in promoting the maturation and differentiation of RPE cells.41
The interaction between genetic mutations and epigenetic modifications is an emerging concept of great importance. Environmental stressors, such as smoking, ultraviolet radiation, and air pollution-induced oxidative stress, can work together with genetic predispositions to increase the risk of disease onset and development.11 For example, mutations in photoreceptor-specific genes are often associated with altered methylation patterns that further inhibit gene expression, thereby accelerating degeneration.42,43 On the other hand, preclinical models have shown that epigenetic therapies aimed at reversing these maladaptive modifications hold promise, highlighting the therapeutic potential of targeting epigenetic dysregulation in retinal degeneration.44
Oxidative stress and mitochondrial dysfunction
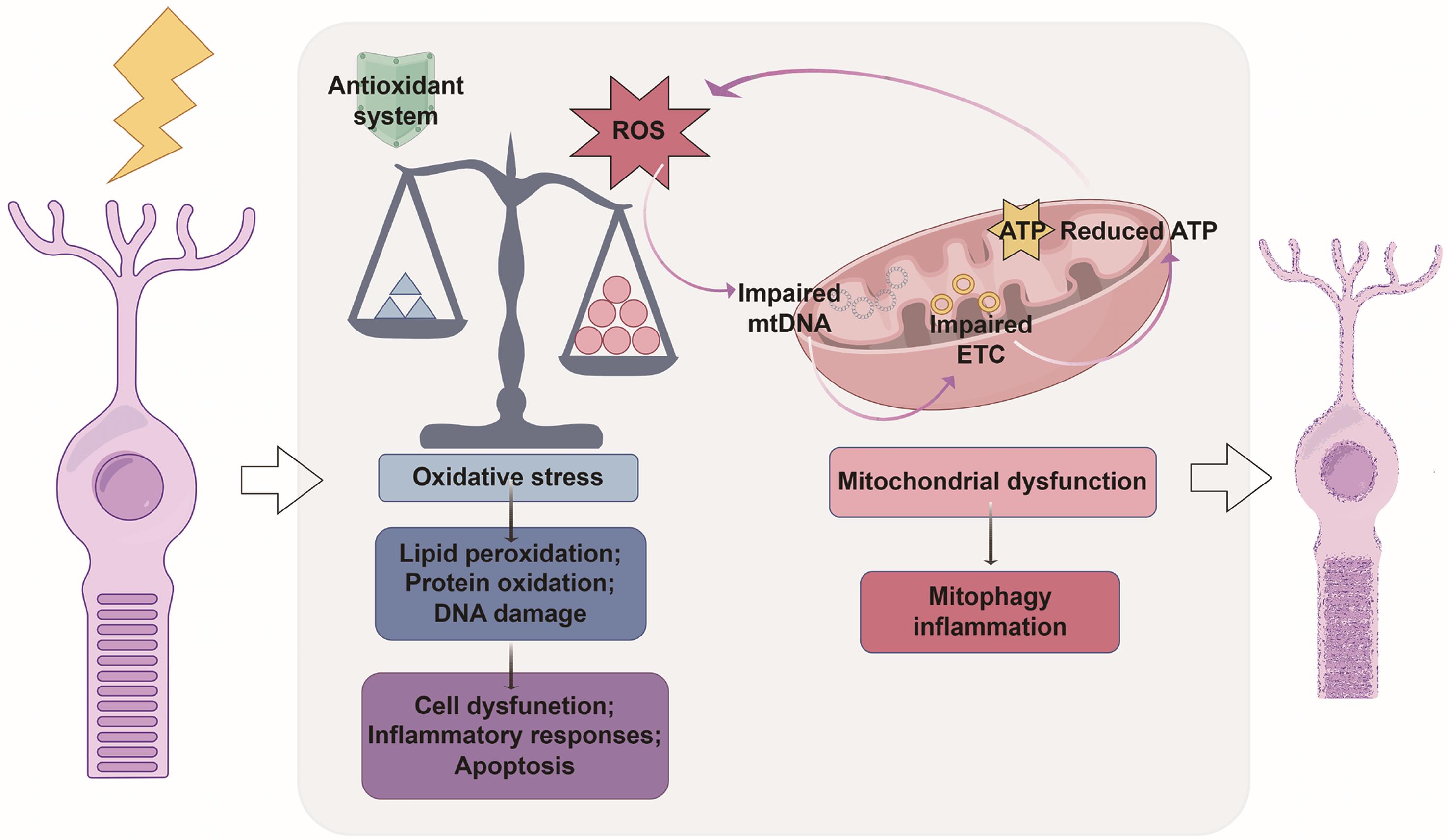
Retinal degeneration arises from a complex interplay between oxidative stress and mitochondrial dysfunction, both of which critically compromise retinal cell survival (Fig. 4). The retina, characterized by high metabolic activity and oxygen consumption, is especially susceptible to the accumulation of ROS, which can trigger lipid peroxidation, protein oxidation, and DNA damage.13 When pathological conditions cause ROS production to exceed the capacity of the retina’s endogenous antioxidant defenses, retinal apoptosis is initiated.45 Mitochondria, which are central to both adenosine triphosphate generation via oxidative phosphorylation and ROS production, play a key role in this process.46 Under normal conditions, a small amount of electron leakage from the electron transport chain leads to low-level ROS formation.47 However, in the context of retinal degeneration, mitochondrial dysfunction—characterized by impaired electron transport, diminished adenosine triphosphate production, and increased electron leakage—significantly elevates ROS generation.48,49 Additionally, chronic oxidative stress upregulates the expression of the fission protein dynamin-related protein 1 and downregulates the fusion-related protein optic atrophy 1, leading to mitochondrial fragmentation, leading to mitochondrial fragmentation. This disruption of the mitochondrial network integrity facilitates the release of cytochrome c and other proapoptotic factors, thereby increasing the likelihood of apoptosis.50
The conceptual diagram depicts the intricate crosstalk between oxidative stress and mitochondrial dysfunction during retinal degeneration, which collectively compromises cell survival. ATP, adenosine triphosphate; ETC, electron transport chain; mtDNA, mitochondrial DNA; ROS, reactive oxygen species.
Mitophagy, the selective removal of damaged mitochondria via autophagy, is essential for mitigating mitochondrial dysfunction.12 Under physiological conditions, mitophagy preserves mitochondrial quality and function. However, in retinal degeneration, excessive ROS and mitochondrial damage impair this protective process. As dysfunctional mitochondria accumulate, ROS production further increases, and energy metabolism becomes increasingly disrupted. These factors create a deleterious feedback loop that accelerates photoreceptor cell death.51,52 Moreover, the build-up of mitochondrial DNA (mtDNA) mutations is another key factor. Human induced pluripotent stem cell–derived RPE cells harboring mitochondrial DNA (mtDNA) mutations associated with mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes exhibit high heteroplasmy, resulting in deficits in mitochondrial function and mitophagy. These findings implicate mtDNA mutations in the disruption of mitochondrial quality control and in the promotion of AMD-like pathology.53
Oxidative stress activates various signaling cascades that amplify retinal damage. ROS can stimulate the MAPK pathway, leading to the phosphorylation of transcription factors that upregulate pro-apoptotic and pro-inflammatory genes.54,55 In parallel, oxidative stress disrupts calcium homeostasis; elevated intracellular calcium levels can trigger the opening of the mitochondrial permeability transition pore, resulting in mitochondrial swelling, depolarization, and the activation of cell death pathways.56 This calcium dysregulation further compromises mitochondrial function and increases cellular susceptibility to oxidative damage. Excessive ROS also triggers adaptive metabolic reprogramming in retinal cells. Under high oxidative conditions, cells may shift their metabolism from oxidative phosphorylation toward glycolysis and the pentose phosphate pathway to generate additional reducing equivalents such as nicotinamide adenine dinucleotide phosphate in an effort to combat ROS.48,57 Despite these compensatory shifts, persistent oxidative stress continues to compromise cellular viability, ultimately leading to irreversible retinal damage.
Inflammation and immune dysregulation
Inflammation serves as a pivotal molecular mechanism in retinal degeneration, originating from intricate interactions between resident immune cells and retinal tissue that ultimately lead to photoreceptor and RPE cell dysfunction.14 In the context of retinal degeneration, chronic inflammatory responses are characterized by abnormal microglial activation, peripheral immune cell infiltration, and cytokine network dysregulation. These factors collectively disrupt retinal homeostasis and expedite cell death.58,59 Recently, Yu et al.59 unveiled a distinctive microglial profile, marked by galectin-3 upregulation at atrophic sites in both mouse models and human AMD. These findings demonstrated that deletion of microglial galectin-3 resulted in phagocytosis defects, increased photoreceptor death, RPE damage, and vision loss, highlighting its protective role. Furthermore, Trem2 signaling was shown to direct microglial migration to atrophic sites and induce galectin-3 expression, with pharmacological Trem2 activation protecting in a galectin-3-dependent manner.59 Another study indicated that nascent RPE inflammation cascades to involve microglial activation and photoreceptor degeneration with monocyte infiltration and that inflammation drives severe, early-onset photoreceptor degeneration associated with Mertk loss of function.60
Neutrophils have been implicated in chronic retinal inflammation, according to recent research. In a retrospective case-control study, He et al.61 observed a significantly greater neutrophil-to-lymphocyte ratio (NLR) in the peripheral blood of RP patients than in that of control patients with only age-related cataracts. Moreover, the NLR was positively correlated with the degree of visual function impairment, implying that systemic neutrophil-mediated inflammation contributes to RP progression. Additionally, Fan et al.62 reported that neutrophils cocultured with the adult retinal pigment epithelial cell line-19 in a laser-induced choroidal neovascularization (CNV) mouse model markedly increased the secretion of various pro-inflammatory cytokines and induced DNA double-strand breaks, leading to S-phase arrest in RPE cells and facilitating CNV formation, thus revealing the crucial pro-inflammatory role of neutrophils in wet AMD pathogenesis. In addition to microglia and neutrophils, the RPE also plays a dual role. It supports photoreceptor function and modulates local immune responses. Under physiological conditions, the RPE serves as an immunoregulatory barrier. However, under conditions of oxidative stress and ER dysfunction, the expression of key immunomodulatory molecules such as interleukin-1 receptor-associated kinase M in the RPE decreases, resulting in increased inflammatory cytokine secretion and aggravated outer retinal degeneration.63
In addition to cellular players, complex signaling pathways integrate environmental stress signals with intrinsic immune responses. The cyclic GMP-AMP synthase (cGAS)-STING pathway, which is activated by cytosolic DNA fragments, has emerged as a critical mediator of innate immune responses. In models of light-induced retinal degeneration, aberrant cGAS-STING activation in microglia and infiltrating macrophages is correlated with elevated proinflammatory cytokine expression and accelerated photoreceptor apoptosis.64,65 Moreover, prolonged ER stress can potentiate inflammatory responses by activating nuclear factor kappa B and other transcription factors that upregulate cytokine production, linking protein misfolding with immune dysregulation in a self-perpetuating cycle.17,66
Protein misfolding, ER stress, and autophagy
High metabolic activity and rapid protein turnover render retinal neurons and RPE cells exceptionally susceptible to proteostatic disturbances. Aberrant protein folding and aggregation trigger ER stress, activating the UPR as an initial protective mechanism to restore cellular homeostasis.67 However, chronic ER stress converts sustained UPR signaling from cytoprotective to pro-apoptotic, significantly contributing to retinal degeneration in diseases such as AMD and RP.15–17 The accumulation of misfolded proteins in the ER, particularly in RPE cells, has emerged as a key molecular event in retinal degeneration. The UPR is mediated through key transducers, including inositol-requiring enzyme 1 alpha, protein kinase R-like endoplasmic reticulum kinase, and activating transcription factor 6. Recent conditional knockout studies have shown that deletion of inositol-requiring enzyme 1 alpha in rod photoreceptors does not affect early retinal development but leads to significant photoreceptor loss and functional decline in aged retinas, underscoring the importance of properly regulated ER stress signaling in maintaining retinal integrity.68 These data emphasize that while the UPR initially plays a protective role, its prolonged activation due to persistent accumulation of misfolded proteins may ultimately trigger cell death.
In addition to UPR activation, changes in autophagy, which is key for breaking down misfolded proteins and damaged cell parts, are crucial in retinal degeneration. Impaired autophagy often occurs early in disease. For example, in the rd10 mouse model of RP, clear changes in autophagy markers such as p62 and LC3 are observed even before photoreceptor degeneration is obvious. These findings suggest that defective autophagy contributes to the formation of harmful protein aggregates and exacerbates ER stress.18 Additionally, abnormal aggregation of RNA-binding proteins (RBPs) and stress granule formation are closely linked to disrupted autophagy and ER stress in the degenerating retina.18,19 Chaperone-mediated autophagy (CMA) is also vital for retinal proteostasis. Orally bioavailable small molecules selectively activate CMA in vivo by stabilizing the retinoic acid receptor α–NCOR1 complex, fine-tuning retinoic acid receptor alpha-dependent transcription.69 These activators preserve CMA activity during aging and markedly reduce photoreceptor degeneration in an RP mouse model, highlighting a novel therapeutic strategy for retinal diseases.
Ubiquitination, a critical posttranslational modification, regulates protein degradation via both the ubiquitin-proteasome system (UPS) and autophagy. Disruptions in ubiquitin-mediated proteolysis have been implicated in retinal degeneration. In such cases, impaired clearance of misfolded proteins exacerbates cellular stress and promotes neurodegenerative cascades.70 Therefore, a finely tuned balance between ubiquitination and autophagic degradation is essential for maintaining retinal protein homeostasis. Any imbalance may increase the susceptibility of retinal cells to dysfunction and death.70,71 Importantly, cellular stress responses in retinal cells are not confined solely to the ER. For example, ER stress-induced disruption of autophagy can adversely affect mitochondrial quality control, thereby further amplifying the degenerative process.72,73
Vascular abnormalities and changes in the extracellular matrix
Vascular anomalies are crucial in driving retinal degeneration. A recent study revealed that patients with advanced RP exhibit reduced macular vessel density, with notable differences observed in all four quadrants of the deep capillary plexus and three quadrants of the superficial capillary plexus.21 Another retrospective study employed optical coherence tomography angiography to investigate macular vascular abnormalities in patients with macular dystrophies and RP compared to healthy controls. The findings revealed that patients without edema presented minimal or no alterations in foveal avascular zone (FAZ). In contrast, RP patients with edema exhibited significantly reduced FAZ dimensions—both vertically and horizontally—as well as a smaller FAZ surface area in the superficial vascular complex. Meanwhile, the FAZ in the intermediate capillary complex was markedly enlarged.74 Cross-sectional research by Overbey et al.75 provided a comprehensive database for choriocapillaris flow deficit percentage (CCFD%) across dry AMD stages via swept-source optical coherence tomography angiography. The CCFD% increases with the severity of AMD, an incomplete RPE, outer retinal atrophy, and subretinal drusenoid deposits (SDDs), particularly in the early and intermediate stages, as well as with the size of RPE atrophy.75 These findings support CCFD% as a valuable clinical and research biomarker and underscore the need for longitudinal studies to confirm its prognostic value.75 Abdolrahimzadeh et al.76 demonstrated significant choriocapillaris damage in early AMD, particularly in eyes with SDDs. The central macular choriocapillaris flow area in the SDD group was significantly lower (p ≤ 0.001) than that in the healthy control group, and there was a trend toward reduced vessel density in the superficial capillary plexus and deep capillary plexus in the SDD and conventional drusen groups.76
ECM changes are key factors driving retinal degeneration. DiCesare et al.20 demonstrated that glycogen synthase kinase 3 inhibitors significantly decrease ECM protein expression in the outer retina and RPE basement membrane, reducing basal deposits and inhibiting AMD-like pathology in STZ-induced mice and in vitro RPE cells. These findings highlight the glycogen synthase kinase 3-ECM axis as a potential therapeutic target for AMD. Obasanmi et al.77 identified granzyme B (GzmB) as a novel therapeutic target for neovascular AMD (nAMD). GzmB, a serine protease, is increased in the RPE and choroidal mast cells of aging and nAMD eyes, promoting ECM degradation, inflammation, and angiogenesis. In vitro and in vivo experiments demonstrated that inhibiting GzmB or preventing mast-cell degranulation reduced choroidal angiogenesis and CNV lesions. Thus, targeting GzmB could be a new approach to suppress CNV in nAMD. Navneet et al.78 highlighted the role of elastase enzymes in AMD progression. These enzymes degrade elastin in the ECM, compromising Bruch’s membrane contributing to CNV. Elevated elastase activity was observed in both AMD models and patient cells. Treatment with A1AT, an elastase inhibitor, reduced CNV lesions and restored RPE integrity in mice, suggesting that it could modify AMD progression by stabilizing the ECM.
Lipid metabolic dysregulation
Lipid metabolism is not only the energetic foundation for maintaining retinal structure and function but also a key driver in the pathogenesis and progression of various RDDs.79,80 As one of the most metabolically active tissues in the body, retinal homeostasis relies heavily on precise metabolic regulation. Photoreceptors are particularly dependent on lipid metabolism: their outer segments contain high levels of polyunsaturated fatty acids (PUFAs), especially docosahexaenoic acid (DHA), which are crucial for membrane fluidity, visual signal transduction, and synaptic function.81 Lipid metabolism supports the continuous renewal of photoreceptor outer segment membranes, which undergo daily turnover through a coordinated cycle of synthesis by photoreceptors and phagocytosis by the RPE.82 Cholesterol, phospholipids, and sphingolipids are also tightly regulated to preserve photoreceptor integrity, intercellular signaling, and visual cycle function.83
Defects in lipid transport or cholesterol/phospholipid metabolism can result in abnormal lipid accumulation within cells and in extracellular matrices, such as Bruch’s membrane and drusen/subretinal drusenoid deposits, leading to RPE stress, complement activation, inflammatory cascades, and ultimately photoreceptor degeneration.84–86 For example, ABCA4 loss-of-function, associated with Stargardt disease, has been shown in recent lipidomic studies to cause intracellular and extracellular accumulation of A2E and retinoid intermediates in RPE cells. This is accompanied by broader lipidome remodeling and lipid droplet deposition, which exacerbates phototoxicity, oxidative stress, and RPE/photoreceptor cell death, thereby mechanistically linking visual cycle metabolism with cellular toxicity.87 Similarly, Bietti crystalline dystrophy—caused by mutations in CYP4V2, an enzyme critical for PUFA metabolism and membrane lipid turnover—leads to disrupted lipid metabolism and local crystalline deposits, driving progressive RPE and retinal atrophy.88
Photoreceptor membranes, rich in PUFAs, are highly susceptible to lipid peroxidation under conditions of high metabolic demand and light exposure. This generates reactive aldehydes such as 4-hydroxynonenal and malondialdehyde, which damage membrane proteins and mitochondria, thereby amplifying inflammatory responses.89 Concurrently, iron dyshomeostasis accelerates lipid peroxidation via Fenton chemistry, promoting ferroptosis, a lipid peroxidation-dependent mode of cell death, as a central mechanism underlying damage to the RPE and photoreceptors.90 Recent cellular and animal studies have directly linked ferroptosis to AMD and blue light/A2E-induced RPE degeneration, providing a molecular basis for targeted interventions.91
Moreover, dysregulation of specific lipid classes, such as sphingolipids and ceramides, directly impacts cell fate by inducing ER stress, mitochondrial dysfunction, and apoptosis or necrosis-like pathways.92 Tahia et al.93 demonstrated that in a BALB/c mouse model of light-induced retinal damage, systemic administration of L-cycloserine—an inhibitor of ceramide synthesis—after a 30-minute pretreatment significantly reduced pro-apoptotic gene expression, and protected photoreceptors from cell death. These findings support its potential as a novel therapeutic approach for treating RDDs.93
Therapeutic strategies
Cell-based therapies and regenerative medicine hold immense potential for the treatment of RDDs. Advances in stem cell technologies, transplantation techniques, and bioengineering approaches have brought these therapies closer to clinical application. While significant hurdles remain, ongoing research is steadily addressing key challenges related to immune compatibility, cell differentiation, and functional integration. Future developments in gene editing, biomaterials, and personalized medicine may further increase the feasibility and effectiveness of cell-based approaches for retinal regeneration.29,94,95 As research continues to refine these strategies, the goal of restoring vision for patients with currently untreatable retinal disorders is becoming increasingly attainable. Currently available treatments for RDDs are summarized in Table 1, providing an overview of pharmacological, gene-based, cellular, and device-assisted therapeutic options.
Therapeutic strategies for retinal degenerative diseases (RDDs)
| Category | Subcategory | Representative methods/drugs/technologies | Key findings/therapeutic effects |
|---|---|---|---|
| Pharmacological interventions | Anti-inflammatory & immunomodulation | Corticosteroids (triamcinolone, dexamethasone) | Effective for cystoid macular edema in RP |
| Anti-angiogenesis | Anti-VEGF agents (bevacizumab, ranibizumab, etc.) | Systematic review confirms efficacy in DME, RVO-ME, and nAMD; improves BCVA and reduces CMT | |
| Dual-targeted therapy | Faricimab (anti-VEGF + anti-Ang-2) | Comparable BCVA to anti-VEGF, but better anatomical outcomes | |
| Conventional/repurposed drugs | Isopropyl unoprostone, nilvadipine, valproic acid, growth factors | Increase retinal sensitivity or slow IRD progression | |
| Drug repurposing | Metformin | Associated with reduced AMD risk in non-diabetic patients; neuroprotective, antioxidant, mitochondrial-supportive | |
| Antioxidants | N-acetylcysteine | Improves BCVA and cone function in RP patients | |
| Nutritional supplements | DHA, lutein, zeaxanthin, curcumin, vitamin A, zinc, manganese, saffron, safranal, coenzyme Q | Support photoreceptor survival, improve ERG, increase ONL thickness | |
| Gene therapy & molecular approaches | Gene replacement | Luxturna (AAV2-RPE65) | FDA-approved for LCA2; 65% patients show functional vision improvement |
| Gene silencing | siRNA/miRNA (e.g., anti-VEGF) | Under investigation in AMD, glaucoma | |
| Gene editing | CRISPR/Cas9, TALENs, ZFNs | Correct RP/IRD mutations; reverse phenotypes in cells/animal models | |
| Modifier gene therapy | Nr2e3, RORα | Enhance transcriptional networks, retinal homeostasis, slow degeneration | |
| Delivery systems | AAV (RGX-314, AAVv128), liposomes, nanoparticles | Improve transduction efficiency; reduce VEGF injection burden; suppress CNV | |
| Cell-based approaches | Embryonic stem cells (ESCs) | ESC-derived RPE/photoreceptors | Restore partial visual function in animals; limited by rejection and ethics |
| Induced pluripotent stem cells (iPSCs) | iPSC-derived RPE grafts, RPE + progenitor cell co-transplantation | Long-term survival and benefit in rodent, porcine, primate models | |
| Mesenchymal stem cells (MSCs) | MSC transplantation, MSC-derived exosomes/miRNAs | Anti-inflammatory, maintain blood–retina barrier, promote visual recovery | |
| Retinal prostheses & nanotechnology | Electronic prostheses | Retinal implants | Restore basic vision in late-stage RP/AMD |
| Nanomaterial-based prostheses | Gold nanoparticle–titania nanowires, tellurium nanowire networks | Restore light responses in blind mice and primates; stable long-term effects | |
| Optogenetic therapy | Microbial opsins | Channelrhodopsins, ReaChR | Confer photosensitivity to inner retinal neurons, partial vision restoration |
| Hybrid stimulation | Optogenetics + electrical stimulation | Enhanced neural responsiveness, reduced latency | |
| Complementary therapies | Electrical stimulation | Transcorneal or transorbital stimulation | Improve blood flow, oxygen consumption; delay visual field loss |
| Acupuncture | Electro-acupuncture | Increase ocular blood flow, reduce RGC injury | |
| Physical activity & lifestyle | Exercise, yoga | Enhance photoreceptor survival, reduce inflammation, improve quality of life | |
| Psychosocial support | Psychological counseling, socioeconomic support | Reduce anxiety/depression, improve vision-related quality of life |
Pharmacological interventions
Pharmacological interventions play crucial roles in managing retinal degeneration, offering ways to slow disease progression and preserve visual function by targeting underlying molecular mechanisms. Many studies have focused on agents that address key pathogenic processes, such as abnormal angiogenesis, inflammation, oxidative stress, and neurodegeneration.
Anti-inflammatory therapies, along with immunomodulation, are critically important in managing the inflammatory processes associated with retinal degeneration. Intravitreal injections of corticosteroids such as triamcinolone and dexamethasone have proven effective in treating cystoid macular edema, which is a common complication in patients with RP.96 Similarly, anti-VEGF therapy has become a cornerstone for managing diabetic macular edema, macular edema related to retinal vein occlusion (RVO), and nAMD. The systematic review by Aldokhail et al.,97 which included 18 studies (ranging from randomized controlled trials to prospective studies, retrospective analyses, and observational studies), demonstrated that anti-VEGF therapy was effective across all three conditions. Different proportions of patients experienced improvements in best-corrected visual acuity (BCVA) and reductions in central macular thickness (CMT). Specifically, the proportion of patients with ≥15 ETDRS letters in DME ranged from 18.1% to 44.8%, whereas the mean changes in BCVA in RVO-related ME and nAMD patients were between +4.2 letters and +21.4 letters. The reduction in CMT in DME and RVO-related ME ranged from 183.1 µm to 294 µm. Pharmacological approaches also encompass dual-targeted strategies. A systematic review and meta-analysis comparing faricimab (a bispecific antibody targeting both VEGF and angiopoietin-2) with conventional anti-VEGF agents revealed that faricimab provided comparable BCVA improvements and better anatomical outcomes, such as reduced central foveal and choroidal thickness.24 Such dual-targeted approaches may yield greater benefits by simultaneously modulating angiogenic and inflammatory processes. Additionally, over the past 10–12 years, traditional medications such as the prostaglandin F2 α-agonist isopropyl unoprostone, the calcium channel blocker nilvadipine, valproic acid, and growth factors have been utilized to increase retinal sensitivity or slow the progression of inherited retinal diseases (IRDs).98
Emerging evidence advocates the repurposing of drugs with established safety profiles to protect the retina. A recent case-control study indicated that metformin, a widely used antidiabetic agent, was associated with reduced odds of developing AMD in non-diabetic patients.99 Metformin appears to have neuroprotective effects by modulating inflammatory pathways, reducing oxidative stress, and enhancing mitochondrial function. In addition, N-acetylcysteine, which is commonly used for pulmonary and psychiatric disorders, can directly scavenge free radicals, thereby reducing oxidative damage. This effect may enhance cone function and survival in RP. One study administered different doses of N-acetylcysteine (600 mg to 1,800 mg) twice daily to RP patients for 12 weeks and then three times daily for another 12 weeks, leading to a significant improvement in the mean BCVA.100
The therapeutic potential of specific nutrients for RDDs is increasingly being revealed. DHA is recognized for its critical role in retinal development and maintenance, and is thought to promote photoreceptor health because of its antioxidant properties.101 DHA levels are reduced in both mouse RP models and human RP patients.102 Lutein, a xanthophyll and the primary carotenoid that accumulates in the human macula to form macular pigment, has antioxidant properties that may benefit retinal health. Studies on animal models have investigated their protective effects against RP, yielding promising results in preventing photoreceptor degeneration. Treatment with lutein and zeaxanthin, another xanthophyll, results in larger a-wave and b-wave amplitudes in dark-adapted electroretinography (ERG), as well as larger b-wave amplitudes in light-adapted ERG.103 Moreover, lutein administration has been associated with a significant increase in outer nuclear layer thickness in mice.104 Curcumin, a bioactive compound derived from the plant turmeric (Curcuma longa), has garnered interest because of its potential multifaceted pharmacological effects, including anti-inflammatory, antioxidant, and neuroprotective properties. Curcumin suppresses the production and release of pro-inflammatory cytokines, chemokines, and enzymes involved in retinal inflammation. Preclinical studies have reported that through these mechanisms, curcumin contributes to the preservation of the retinal structure, increased thickness of the outer and inner nuclear layers, and improved ERG responses.105 Moreover, promising results have been obtained in assays involving dietary supplementation with antioxidant compounds such as vitamin A, zinc, manganese, saffron, safranal, and coenzyme Q.106,107
Gene therapy and molecular approaches
Gene therapy has ushered in a new era in the treatment and potential cure of diseases, offering hope to millions of people impacted by inherited disorders or harboring disease-causing mutations.108 The development and application of gene therapy for RDDs have consistently been at the cutting edge of translational medicine. Retinal gene therapy methods differ depending on the type of mutation and may involve gene replacement/augmentation, silencing/editing of the mutated gene, or supplying a modifier gene that influences upstream or downstream pathways from the defective gene to enhance cellular function.
Gene replacement directly supplies a functional copy of a damaged or nonfunctional gene to increase functional protein production. It is ideal for monogenic recessive inherited diseases. For example, CEP290 gene mutations are a leading cause (15–20%) of Leber congenital amaurosis.109 Voretigene neparvovec (VN), the first U.S. Food and Drug Administration (FDA)-approved gene replacement therapy marketed as Luxturna, is used to treat severe Leber congenital amaurosis type 2. In a phase III trial, 29 patients with RPE65-linked retinal dystrophy received subretinal injections of Luxturna. After one year, 65% of participants showed functional vision improvement in the multiluminance mobility test.110 Gene silencing uses small interfering RNA (siRNA) to break down sequence-specific mRNAs, eliminating the product of a faulty gene. Gene silencing with siRNAs or microRNAs targeting VEGF is being developed for AMD, glaucoma, and other ocular diseases.111–113 Several clinical trials utilizing targeted gene silencing techniques are currently underway.114–116
Gene editing involves correcting individual mutations or reducing the expression of mutated proteins in a targeted way. This technique fixes gene mutations or decreases the expression of faulty proteins to change the disease state. Several gene-editing techniques have been developed, including clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9), transcription activator-like effector nucleases (TALENs), zinc finger nucleases, and meganucleases.117 Among these, CRISPR/Cas9 is the most well known and has shown potential in gene therapy. In 2016, Bakondi et al.118 first demonstrated in vivo functional ablation of an inherited dominant mutation via CRISPR/Cas9 by targeting a mutant Rho allele in a rat model of autosomal dominant RP. Recently, Chen and colleagues created a human iPS cell line (CSUASOi006-A) from an RP patient with a pre-mRNA processing factor 8 (c. C5792T) mutation.119 They used CRISPR/Cas9 to correct the c.5792C > T mutation in pre-mRNA processing factor 8 and generated an isogenic control cell line (CSUASOi006-A-2), providing a key cellular resource for RP research. Siles et al.120 precisely corrected seven hiPS cell lines from IRD patients with mutations in ABCA4, BEST1, PDE6A, PDE6C, RHO, or USH2A via CRISPR/Cas9 and TALENs. The corrected clones reversed the disease-associated phenotype in retinal cellular models,120 strengthening the study and application of gene-editing-based IRD treatments. Modifier gene therapy can affect pathways downstream or upstream of multiple defective genes, addressing clinical phenotypes without genetic diagnosis in a mutation-agnostic way. Li et al.121 found that therapy with the nuclear hormone receptor gene Nr2e3 reduced retinal degeneration. They reported an increase in photoreceptor cells, improved electroretinogram, and a molecular reset of key transcription factors and gene networks, enhancing retinal homeostasis in diseased tissue. Chang et al.122 discovered that retinoic acid-related orphan receptor α, which acts as a genetic modifier, can rescue retinal degeneration in mouse models of Stargardt disease and dry AMD.
Retinal gene therapy employs a variety of delivery vehicles, including adenovirus, adeno-associated virus (AAV), retroviral and lentiviral vectors, naked DNA/RNA, synthetic polymers, niosomes, and lipid-based carriers such as liposomes and lipid nanoparticles. The administration routes for these delivery vehicles include subtenon, subconjunctival, subretinal, and suprachoroidal ocular implants. Optimizing vector delivery methods and dosing strategies is key to the success of gene therapy. Luo et al.123 introduced a novel AAV capsid, AAVv128, which has increased transduction efficiency for photoreceptors and RPE cells and broader retinal tissue distribution in various animal models after intraocular injection. Notably, suprachoroidal delivery of the AAVv128-antiVEGF vector effectively suppressed Grade IV lesions in a laser-induced CNV NHP model of nAMD. Campochiaro et al.124 evaluated the safety and efficacy of RGX-314, an AAV8 vector expressing an anti-VEGF-A antibody fragment, which was administered via subretinal injection in nAMD patients. In this phase 1/2a trial, 42 participants received single subretinal injections of RGX-314 across multiple doses (3 × 109 to 2.5 × 1011 genome copies per eye) and were followed for 2 years. The results showed that RGX-314 was generally well tolerated, with no clinically significant immune responses. At doses ≥6 × 1010 genome copies, most patients maintain stable/improved vision and retinal thickness with few or no additional anti-VEGF injections needed.
Cell-based approaches
Cell-based therapies have come to the forefront as promising solutions to address the complex challenges of retinal degeneration. These therapies have the potential to restore lost retinal tissues or repair damaged ones, to restore vision or at least prevent further deterioration. Over the years, extensive preclinical and clinical trials have been carried out to explore the efficacy of cell therapy for RDDs. A variety of cell types, including embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), and progenitor cell-derived cells, have been employed in these trials to treat different retinal conditions and enhance functional outcomes. Such research has been conducted across a range of animal models and has addressed diseases from RP to AMD, glaucoma, and general retinal degeneration.
One significant benefit of employing ESCs for degenerative diseases is their ability to indefinitely differentiate into virtually any cell type. Wu and colleagues effectively induced rat ESCs to differentiate into RPEs and photoreceptors, and retinal transplantation in RCS rats resulted in the restoration of visual function.125 In RCS rats, following the transplantation of human embryonic stem cell (hESC)-derived RPEs, an improvement in visual performance was observed compared to untreated controls.126 Nevertheless, although mouse ESCs integrate into mild retinal degeneration models and exhibit mature morphologies with photoreceptor markers, in severe retinal degeneration models, the transplanted cells survive but do not acquire mature morphological characteristics.127
Although ESC-based replacement therapy is highly important for retinal regeneration, its application is hindered by immune rejection, tumor formation, and ethical issues. Sharma et al.128 developed clinical-grade iPSCs from AMD patients without oncogene mutations and differentiated them into RPE patches on biodegradable scaffolds. This allows the cells to integrate into rodent and porcine models of AMD-like eye diseases.128 Furthermore, Salas et al.129 reported that a combination of hiPSC-derived RPE cells and retinal progenitor cells was more effective at preserving intrinsic photoreceptors and visual function in both early- and late-stage disease degeneration than transplanting either cell type alone. The long-term effectiveness of human iPSC-derived cells after transplantation has also been demonstrated. Human iPSC-derived retinal grafts have been shown to survive for up to five months in rats and for two years in monkey models.130,131 Nonetheless, in porcine models mimicking advanced AMD, subretinal transplantation of hiPSC-derived RPE cells was less effective in atrophic regions than in healthy areas.132
Another approach that supports autologous cell transplantation and reduces immune rejection involves the use of MSCs. Owing to their anti-inflammatory properties, growth factor-producing ability, and role in promoting tissue regeneration, MSCs are well-suited for retinal degenerative cell therapies.133 Notably, MSCs can differentiate into photoreceptor-like cells, amacrine cells, bipolar cells, and RPE cells.134–136 Recent studies have demonstrated that intravitreal injections of MSCs have protective effects on the retina and can enhance visual function.137,138 Additionally, MSCs can serve as biological patches to preserve the blood-retinal barrier, thereby promoting functional recovery following retinal ischemia/reperfusion.139 Moreover, MSC-derived exosomal miR-125b-5p has been shown to suppress retinal microvascular endothelial cell ferroptosis in diabetic retinopathy.140 Additionally, miR-125a-5p in small extracellular vesicles derived from MSCs alleviates Muller cell injury in diabetic retinopathy by modulating mitophagy through the PTP1B pathway.
Retinal prostheses and nanotechnology
Retinal prostheses aim to provide functional vision for those suffering from severe vision loss. Their effective operation depends on a posterior visual pathway that is reasonably well preserved, including the optic nerve, lateral geniculate nucleus, and visual cortex. For individuals in the advanced stages of retinal degenerative conditions such as RP and AMD, retinal implants serve as a viable option.141,142 Owing to their tunable optoelectronic characteristics, high surface-to-volume ratios, and favorable tissue compatibility, nanomaterials provide promising platforms for next-generation retinal prostheses.143 Recently, Yang et al.144 explored subretinally implanted gold nanoparticle-coated titania nanowire arrays as artificial photoreceptors. Tests in mice and monkeys with induced photoreceptor degeneration showed that arrays have advanced spatial and temporal resolution in ex vivo retinas. In blind mice, they improve visual acuity and help detect certain stimuli. In monkeys, long-term stability and positive impacts on the primary visual cortex were observed. Nie et al.145 demonstrated that intravitreal injection of anti-Thy1-conjugated near-infrared-resonant gold nanorods enables targeted photothermal activation of bipolar cells via a 20 µm-patterned near-infrared (NIR) laser scan, eliciting robust visual cortex responses in both wild-type and blind mouse models without systemic toxicity or retinal damage, thereby offering high-resolution, wide-coverage, and a minimally invasive strategy for customizable vision restoration. Wang et al.146 engineered a subretinal nanoprosthesis composed of tellurium nanowire networks (TeNWNs) capable of transducing both visible and near-infrared II light into electrical impulses. When implanted in blind mice, these TeNWNs reinstated the pupillary light reflex and supported visually guided learning under illumination at visible wavelengths and 1,550 nm. In nonhuman primate studies, TeNWNs evoked strong neural responses originating from the retina.
Optogenetic therapeutic approaches
Groundbreaking advances in optogenetics are driving the development of novel therapeutic approaches to restore vision in individuals suffering from RDDs such as RP. Optogenetics operates by leveraging light-sensitive proteins to make surviving retinal neurons responsive to light, with a primary focus on secondary and tertiary neurons in the retina.147 This allows these neurons to take over the function of the degenerated photoreceptors. Through the use of microbial opsins such as channelrhodopsins, researchers can turn inner retinal neurons into photosensitive cells. Consequently, these neurons can respond to light and restore some visual function even after photoreceptor loss has occurred. Ng et al.148 conducted a retrospective analysis of retinal structure in patients with late-stage IRD to evaluate its suitability for optogenetic gene therapy. In this study, 36 patients (54 eyes) with late-stage IRD were categorized into three groups based on clinical phenotype and history. Spectral-domain optical coherence tomography was employed to analyze structural parameters, including subfoveal thickness and individual inner layers. The results revealed that 46.3% of the degenerated retinas still retained some inner retinal layers or exhibited thickening of the inner nuclear layer. These findings suggest that cell-specific optogenetic therapy may be advantageous. In contrast, patients with unclear or disrupted inner layers might require non-cell-specific approaches that target all surviving neurons. Rohet et al.149 investigated the hybrid approach of combining optogenetic and electrical stimulation to decrease optical power and enhance the effectiveness of retinal stimulation. Compared with optogenetic stimulation alone, hybrid stimulation with a 10 µA square pulse markedly increased spiking activity and reduced latency across all light intensities in wild-type mice. Rodgers et al.150 showed that while introducing the optogenetic protein ReaChR into depolarizing (ON) bipolar cells or retinal ganglion cells (RGCs) in retinally degenerate mice restores visual responses with significant fidelity, targeting ON BCs results in more favorable outcomes, including more diverse and reproducible responses, better-preserved contrast sensitivity and temporal frequency tuning, and less disruption to the visual feature selectivity of individual RGCs than does targeting RGCs directly, thus highlighting that ON BC targeting yields a richer visual code closer to that of wild-type mice.
Complementary therapies
Electrical stimulation (ES) is a non-pharmacological method that delivers microcurrents to target tissues. These microcurrents induce biochemical effects on cells, potentially preserving or restoring vision.151,152 In a patient cohort monitored for up to one year, increased ES was linked to a trend toward preventing visual field loss and enhancing photopic ERG b-wave amplitudes, suggesting an impact on the cone photoreceptor system, which aligns with preclinical biomarker studies.153 Bittner et al.154 reported a significant improvement in retinal blood flow (RBF) in macular capillaries after six weeks of ES treatment. Half a year following transcorneal electrical stimulation (TES) therapy, there was an increase in the mean retinal arteriolar oxygen saturation compared to baseline, whereas the venular saturation decreased, indicating that TES treatment for RP results in increased oxygen consumption in the retina.155
Acupuncture, a vital element of complementary and alternative medicine, has been increasingly applied to treat a range of conditions, including pain, neuropathy, migraine, and insomnia.156 Bittner et al.154 reported that electroacupuncture significantly increased the mean flow velocity of the retrobulbar central retinal artery after two weeks and increased the RBF after one month of treatment compared with controls. Wang et al. revealed that electroacupuncture might reduce RGC injury by modulating the lncRNA-XR_002789763.1/miR-342-5p axis, activating the PINK1/Parkin pathway, and promoting Mfn2 ubiquitination.157
Physical activity is widely acknowledged for its positive impact on overall health and well-being. Preclinical studies have demonstrated that in RP mouse models, exercise exerts a beneficial effect on retinal degeneration by reducing vision loss and retinal damage. This involves an increase in the number of cone cells,158 a reduction in photoreceptor loss, and a decrease in retinal inflammation.159 In a preliminary study, physical activity was found to enhance self-reported visual function and quality of life in patients with RP.160 Additionally, Jiang and colleagues investigated the relationship between retinal microcirculatory responses and improvements in cognitive function in Parkinson’s disease patients following yoga training. Their results indicated that enhanced RBF and increased retinal capillary perfusion density in the superficial vascular plexus were associated with improved performance on the Trail-making A test. Furthermore, changes in capillary perfusion density within the retinal vascular network were linked to better scores on the Hopkins Verbal Delayed Recall test.
Among individuals with retinal dystrophies, the interplay between psychological aspects such as anxiety, fear, and decreased vision-related quality of life can aggravate the condition, establishing a negative feedback loop where psychological distress may impact the disease’s trajectory. RP and progressive visual disability can also lead to significant economic burdens on patients, affecting healthcare expenses and personal support costs and resulting in a loss of working hours, work quality, and income.161,162
Challenges
Despite notable progress in elucidating the molecular underpinnings of retinal diseases, the rapid advancement of gene therapies, cell-based interventions, and regenerative medicine for retinal degeneration has outpaced traditional regulatory frameworks. Advancing treatments for retinal degenerative disorders requires overcoming a multitude of challenges, including biological complexity, limitations in preclinical modeling, delivery barriers, ethical considerations, and the need to balance innovation with rigorous risk management. Below, we detail these obstacles and their implications for advancing therapies.
Biological complexity and patient heterogeneity
Retinal degenerative disorders exhibit profound genetic and phenotypic heterogeneity, posing a formidable challenge to any universal therapeutic approach. To date, mutations in more than 330 genes have been linked to IRDs, each giving rise to distinct clinical phenotypes and rates of progression.163,164 In addition to monogenic causes, epigenetic alterations further diversify disease mechanisms, increasing the complexity of pathogenesis.165 Furthermore, both intra- and interfamilial variability in severity and trajectory impedes the reliable prediction of treatment outcomes.166 Elhusseiny et al.167 investigated multiple affected members within a single pedigree harboring the same pathogenic PRPH2 mutation and reported marked interindividual differences in BCVA and the rate of clinical progression, despite a shared genetic background. Birch et al.168 demonstrated that the type of mutant allele (null vs. expression-modulating) was significantly correlated with phenotypic severity, even among individuals from the same family (p < 0.01).
Limitations of preclinical models
Bridging the divide between animal models and human retinal disease remains a critical translational challenge. Although rodent and porcine models have illuminated key aspects of degeneration, their differing retinal anatomy, immune milieu, and regenerative capacity limit their ability to mirror human pathology.169 Lu et al.170 demonstrated that following subretinal injection of clinical-grade human neural progenitor cells into Yucatan miniature pigs, daily intraperitoneal dexamethasone for two weeks combined with long-term oral cyclosporine A administration was required to maintain graft survival. This contrasts with murine models, where hNPCs with low MHC expression exhibit prolonged survival without extensive immunosuppression, highlighting a more robust intraocular immune rejection response in pigs toward allogeneic or xenogeneic cells. In vitro systems, particularly retinal organoids, offer controlled studies of human tissue but still fall short of replicating the in vivo microenvironment and long-term disease dynamics.171–173 Multiple studies have reported that the inner retinal layers of retinal organoids undergo progressive degeneration during long-term culture (approximately 4–6 months), characterized by a decrease in retinal ganglion cells and thinning of the inner plexiform layer.174–176 These shortcomings contribute to the high attrition rate of preclinically promising therapies in human trials, highlighting the imperative for more representative models and comprehensive translational frameworks.
Delivery and immune response challenges
The delivery of therapies to the retina is complicated by the distinct anatomical and immunological defenses of the eye. Subretinal injections and other invasive delivery methods are necessary to access retinal tissue, but they are accompanied by procedural risks and variability in therapeutic distribution.177–179 The complications associated with subretinal injection include endophthalmitis, elevated intraocular pressure, cataract formation, and vitreous or choroidal hemorrhage.180 However, owing to the limited number of treated patients and the scarcity of long-term follow-up studies, the precise incidence rates of these adverse events remain undetermined. Guest et al.181 reported that the use of different syringe types or injection techniques (e.g., prefilled syringes versus Luer-Lok syringes) can result in up to a 40% discrepancy in injection volume, thereby compromising the uniform distribution of the drug across the vitreous and retinal surfaces. Moreover, immune reactions to viral carriers or transplanted cells can erode treatment gains and provoke inflammation. Clinical variability in AAV-based gene therapies, for example, is partly driven by the host’s clearance of the vector and local immune activation.179,182 Addressing these challenges requires both a smarter vector design to evade immune detection and tailored immunomodulatory strategies to safeguard safety and sustain efficacy.
Balancing innovation with risk management
Innovation in retinal degeneration treatment must be accompanied by a comprehensive risk assessment. Strategies such as gene augmentation therapy and extracellular vesicle-based interventions show exciting potential but bring concerns about immune reactions, unintended targeting, and sustained treatment effects.183,184 In response, regulators are increasingly insisting on in-depth preclinical evidence and early-phase trial data that prove safety over prolonged follow-up periods as well as therapeutic benefit. The development of targeted gene therapies against proteins such as FAM161A highlights the need for precision in vector dose selection and gene regulation to avoid cell toxicity and immune complications.185 The key is to strike the right balance between accelerating approval pathways and enforcing stringent safety requirements. In addition, regulators must adopt a flexible approach to trial requirements, tailoring endpoints to the specific disease mechanisms under investigation while still ensuring that generated data remain rigorous and reproducible.186,187
Ethical challenges in patient safety and informed consent
Patient safety is the foremost ethical priority, as novel retinal therapies have advanced from preclinical studies to human trials. Recent data from stem cell transplantation studies have revealed serious adverse events in inadequately regulated centers, underscoring the necessity of rigorous oversight and transparent inclusion criteria.188,189 Informed consent must likewise evolve to reflect the experimental nature of these treatments, detailing potential off-target effects and the long-term consequences of genetically modified or stem cell-derived products.184,190 Haapaniemi et al.191 reported that prolonged Cas9 expression can elicit p53-dependent growth arrest, which may selectively increase p53-deficient or mutant populations and increase the potential for neoplastic transformation. Ethical considerations also demand attention to equity and access. Advanced gene and cell therapies carry high price tags, making policy interventions and innovative funding strategies essential to ensure broad availability rather than limiting benefits to a privileged few.192,193
Limitations
Despite our efforts to provide a comprehensive overview, this review has several limitations that should be acknowledged. First, as a narrative review, the selection of literature may be subject to bias, and it is possible that not all relevant studies were included. Second, the field of retinal degeneration is highly heterogeneous, encompassing both monogenic inherited retinal dystrophies and multifactorial diseases such as AMD. Given this complexity, certain mechanisms and therapeutic approaches have been emphasized more than others. Third, owing to space constraints, some rapidly evolving areas, including advanced biomaterials, combinatorial nanomedicine strategies, and digital health applications, can only be briefly discussed. Finally, while this review highlights emerging therapeutic strategies, many of these approaches are still in preclinical or early clinical trial stages, and their long-term safety and efficacy remain uncertain.
Conclusions
RDDs pose a significant threat to global vision health, arising from a multifaceted network of genetic and epigenetic alterations, oxidative stress, mitochondrial dysfunction, inflammation, immune dysregulation, protein misfolding, ER stress, impaired autophagy, vascular abnormalities, and ECM disruption. To date, pharmacological treatments, gene- and molecular-based approaches, retinal prostheses, cell-based approaches, optogenetics, TES, and other strategies have all shown promise in clinical trials. Progress in elucidating these molecular mechanisms and advancing therapeutic strategies continues to deepen our understanding of RDDs and holds promise for improving patient outcomes and quality of life. Nevertheless, to realize this objective, several barriers must be surmounted, such as disease heterogeneity, the limitations of preclinical models, and the problem of immune rejection.
Declarations
Acknowledgement
None.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (LQN25H120010, LTGD23H120002); the National Natural Science Foundation of China (82501339, 82101176); the Health Technology Plan Project in Zhejiang Province (2023KY151); and the Science and Technology Project of Wenzhou (Y20220774).
Conflict of interest
The authors have no conflicts of interest to declare.
Authors’ contributions
Article search (YYZ, JYL, JWL), data analysis and interpretation (YYZ), funding acquisition (YYZ, CC, XTL), manuscript writing (YYZ), critical revision of the manuscript (JYL, JWL, CC, XTL), project administration (CC, XTL), technical and material support (CC, XTL). All authors have made substantial contributions to this study and have approved the final manuscript.

 Author information
Author information