Introduction
Central serous chorioretinopathy (CSC) is characterized by serous detachment of the neurosensory retina, along with dysfunction of the choroid and retinal pigment epithelium (RPE).1,2 It is generally considered a self-limiting condition.3 However, the underlying mechanisms of CSC remain incompletely understood.4 The majority of CSC cases resolve spontaneously, with visual acuity typically recovering within three to six months.5 For these patients, the primary management strategy focuses on regular monitoring and follow-up, as well as avoiding known risk factors, such as discontinuing glucocorticoid use and making lifestyle modifications for individuals with Type A personalities.6,7 Type A personality traits, which are characterized by impatience, competitiveness, aggressiveness, and hostility, are often associated with heightened physiological responses.8 For patients experiencing recurrent, chronic, or prolonged cases of CSC, current treatment modalities primarily include laser therapy, surgical interventions, and pharmacotherapy.9 This review aims to provide a comprehensive overview of the pathogenesis, diagnostic approaches, and latest advances in the treatment of complex CSC.
Etiology and pathogenesis
The pathogenesis of CSC remains not fully understood. Current research has identified several risk factors, including corticotropin-releasing hormone (CRH), stress, steroid hormones, hypertension, Type A personality traits, infection with Helicobacter pylori, pregnancy, sleep disturbances, autoimmune diseases, and medication use.10,11 These factors may contribute to the onset and progression of CSC through various physiological and biochemical mechanisms. At present, no specific targeted cells or pathways have been identified for CSC. Schellevis et al.12 performed genome-wide association studies on patients with chronic CSC and found a significant association with a site on the complement factor H gene of chromosome 1. Pathway analysis enriched complement genes, and gene expression analysis suggested the roles of complement factor H, complement factor H-related 1, complement factor H-related 4, CD46, the potassium sodium-activated channel subfamily T member 2, and tumor necrosis factor receptor superfamily member 10a in the disease. This indicates that the complement pathway has potential importance in the pathogenesis of chronic CSC.12 In addition, the fibroblast growth factor receptor plays an important role in maintaining both mature and immature retinal pigment epithelial cells, and may be a potential pathway for CSC.13
CRH
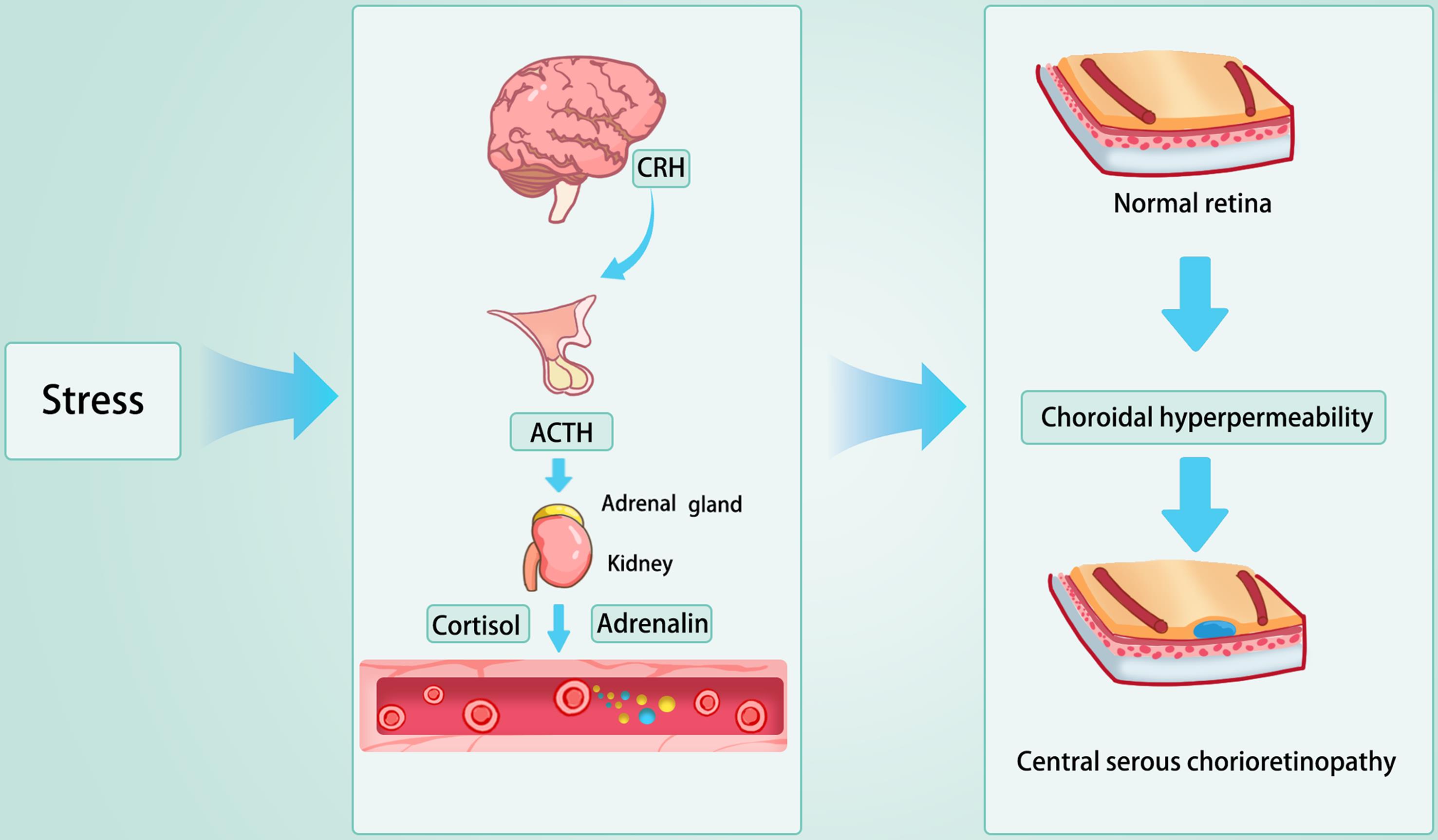
CRH, a polypeptide hormone secreted by the hypothalamus, plays a pivotal upstream role in the pathogenesis of CSC. The primary function of CRH is to stimulate the anterior pituitary gland to secrete adrenocorticotropic hormone, which, in turn, promotes the adrenal cortex to release glucocorticoids such as cortisol.14 Typically, CRH release is regulated by the stress response: under stressful conditions, its secretion increases, leading to elevated levels of adrenocorticotropic hormone and subsequently, glucocorticoids.15 This hormonal cascade is a fundamental component of the hypothalamic-pituitary-adrenal (HPA) axis, which helps maintain homeostasis and regulate the body’s response to stress (Fig. 1).
Under stress conditions, the brain secretes CRH, which subsequently activates the HPA axis. This activation leads to the production of glucocorticoids and mineralocorticoids by the adrenal glands. As a consequence, choroidal permeability is enhanced, and hydrostatic pressure within the choroidal layer increases, resulting in disruption of the retinal pigment epithelium and ultimately causing localized serous retinal detachment. ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; HPA, hypothalamic-pituitary-adrenal.
Recent research has identified a significant association between the expression of the CRH gene and the development of CSC.16,17 Variations in the CRH gene may increase the risk of CSC through two primary mechanisms. Firstly, the expression of CRH is thought to play an important protective role in stress-induced damage. This function is primarily realized through the enhancement of tau protein phosphorylation in the brain and by counteracting oxidative stress-induced neuronal cell death, thus exhibiting neuroprotective properties.18 Studies in transgenic mice that overexpress CRH show that under acute excitatory stress, CRH effectively protects the nervous system from degeneration.19 Conversely, a deficiency in CRH reduces these protective effects, potentially facilitating the onset of CSC. Secondly, CRH expression is closely related to inflammatory cytokines. Animal studies have shown that CRH-deficient mice subjected to stress exhibit elevated levels of inflammatory cytokines, particularly interleukin-6 and tumor necrosis factor-alpha.20 These cytokines contribute to the pathogenesis of CSC by increasing vascular permeability, thereby playing a pivotal role in the disease’s development.
In summary, CRH, as a central factor in the HPA axis, plays a significant role in the pathogenesis of CSC. However, mutations in the CRH gene, combined with environmental stressors, can lead to aberrant responses in the HPA axis. This dysregulated response results in an imbalance in the secretion of glucocorticoids and mineralocorticoids, which may cause individuals with CRH mutations to experience disrupted inflammatory homeostasis, placing the choroid in a state of chronic inflammation. Under the influence of various inflammatory factors, the increased permeability and elevated hydrostatic pressure in the choroidal blood vessels may breach the RPE, ultimately triggering CSC.
Stress
The relationship between CSC and psychological stress has been extensively studied.21–23 Although the precise mechanisms remain incompletely understood, several hypotheses have been proposed to explain this association. One key hypothesis is that psychological stress may influence the onset of CSC by activating the HPA axis. Stress increases glucocorticoid secretion, such as cortisol, and elevated cortisol levels may heighten the risk of subretinal fluid (SRF) accumulation.24 This effect is similar to the impact of glucocorticoid medications in CSC. Furthermore, stress may trigger hyperactivity of the sympathetic nervous system, leading to systemic vasoconstriction, including in the choroidal vasculature.25 This vasoconstriction can impair choroidal circulation, increase choroidal permeability, and ultimately result in SRF accumulation. Additionally, stress may affect the vascular endothelium and the neuroendocrine system, leading to increased vascular permeability, which in turn facilitates the development of CSC.26 This heightened permeability can lead to fluid accumulation beneath the retina, contributing to the pathogenesis of CSC.
Glucocorticoids
The precise mechanisms underlying the association between glucocorticoids, such as cortisol and pharmacological steroids, and CSC remain incompletely understood. However, evidence suggests that glucocorticoids may influence the onset and progression of CSC through several pathways.27 Firstly, glucocorticoids significantly impact fluid homeostasis and vascular function within the body.28 They may increase vascular permeability and choroidal leakage, leading to enhanced exudation in the choroidal vasculature. This choroidal exudation can contribute to the accumulation of SRF, a hallmark pathological feature of CSC.29 Secondly, glucocorticoids might modulate the function of RPE cells.30 They may affect the barrier function of RPE cells and their ability to regulate fluid transport, potentially causing or exacerbating fluid accumulation beneath the RPE. Additionally, glucocorticoids may influence the development of CSC through neuroendocrine pathways.31 By affecting the HPA axis, they could induce an exaggerated stress response, which, in certain cases, has been implicated in the onset of CSC.
Helicobacter pylori
Helicobacter pylori is a common gastric pathogen associated with various gastrointestinal disorders, such as gastritis and peptic ulcers.32 In addition to its gastrointestinal effects, Helicobacter pylori may also be implicated in certain ocular diseases, including CSC, through multiple mechanisms.33 Several hypotheses have been proposed to explain the potential link between Helicobacter pylori and CSC. Firstly, the immune response hypothesis suggests that Helicobacter pylori infection induces a systemic immune reaction, potentially leading to widespread inflammation. This inflammatory response could affect choroidal blood flow and permeability, increasing the risk of CSC development.34 Secondly, oxidative stress is another proposed mechanism. Helicobacter pylori infection may exacerbate oxidative stress, which could impair the cellular functions of the choroid and retina, predisposing individuals to CSC.35 Additionally, the release of vasoactive mediators is considered a possible link. Helicobacter pylori infection may stimulate the release of vasoactive substances, such as nitric oxide and endothelin, which could influence choroidal blood circulation and contribute to the CSC pathogenesis.36 Lastly, alterations in hormonal levels should be considered. Helicobacter pylori infection may trigger chronic stress responses that affect hormonal levels, such as cortisol. These hormonal changes could modify choroidal permeability and pressure, thus creating conditions favorable for CSC development.37 Overall, further research is needed to substantiate these hypotheses and clarify the complex interactions between Helicobacter pylori infection and CSC.
Pregnancy
The relationship between pregnancy and the incidence of CSC may be attributed to significant hormonal fluctuations during gestation.38 The underlying mechanisms can be explained through several pathways. During pregnancy, particularly in the second and third trimesters, there is a marked increase in estrogen and progesterone levels.39 These hormones can enhance the permeability of the choroidal vasculature, potentially leading to the accumulation of SRF and consequently elevating the risk of developing CSC.40 Furthermore, pregnancy is characterized by an increase in systemic blood volume and cardiac output, which may alter choroidal blood flow.41 These changes can impact choroidal pressure and permeability, thereby facilitating the development of CSC.42 Additionally, pregnancy is recognized as a physiological stress condition that may activate the body’s stress response axis, specifically the HPA axis.43 This activation results in elevated secretion of stress hormones, such as cortisol, which has been identified as a risk factor for CSC. Pregnancy-related complications, such as gestational hypertension, can lead to microcirculatory disturbances within the choroid. These vascular changes heighten susceptibility to CSC.
Choroidal hyperpermeability in CSC
Two main theories are commonly discussed in investigations of the mechanisms underlying CSC: the choroidal dysfunction theory, also known as the choroidal hyperpermeability theory,44 and the RPE dysfunction theory, or diffusion theory. The choroidal hyperpermeability theory proposes that certain factors increase the permeability of choroidal capillaries, resulting in significant fluid leakage and subsequent impairment of the RPE.44 This impairment leads to a serous detachment of the RPE.9 As choroidal hydrostatic pressure escalates, the RPE elevates, and mechanical forces disrupt the continuity of the RPE, leading to pigment epithelial detachment and further leakage.45 This progression results in the accumulation of fluid beneath the neurosensory retina.44 Pigment epithelial detachment is considered a compensatory response of RPE function in eyes affected by CSC; isolated leakage or RPE damage is insufficient to cause SRF accumulation unless RPE function decompensates to a critical level.46 An alternative explanation involves impairment of the uveoscleral transport pathway. Factors such as scleral thickening and reduced vortex vein blood flow increase pressure within the choroidal veins and capillaries, impeding lymphatic transport of proteins from the vasculature. These phenomena elevate hydrostatic pressure in the choroidal capillaries and increase extravascular protein concentrations, which may infiltrate the RPE and accumulate beneath the neurosensory retina, carrying fluid with them.47 The precise etiology of choroidal abnormalities remains unclear, but it is thought to be related to the autoregulation of choroidal blood flow. Conversely, the RPE dysfunction theory, or diffusion theory, suggests that certain damaging factors lead to injury of even a few or singular RPE cells. These damaged RPE cells secrete large quantities of ions into the intercellular spaces surrounding photoreceptor cells, attracting choroidal fluid to this area. Initially, fluid transport may occur through cellular channels, but excessive fluid transport can compromise the diffusion barrier in localized areas.48 If the RPE defect is small, early fundus fluorescein angiography (FFA) may reveal minimal leakage points. Rapid fluorescein leakage into areas of disciform detachment indicates substantial and swift fluid passage through the damaged RPE into the subretinal space. These theories provide valuable insight into the complex mechanisms of choroidal and RPE pathophysiology in CSC, though further studies are necessary to clarify the complex interplay of factors contributing to this condition.
Pachychoroid spectrum diseases
The concept of pachychoroid spectrum disorders has emerged within ophthalmology.49 This spectrum encompasses four distinct entities: pachychoroid pigment epitheliopathy (PPE), CSC, pachychoroid neovasculopathy, and polypoidal choroidal vasculopathy.50 These conditions share several characteristics, including increased choroidal thickness, pathological dilation of the choroidal large vessel layer, and thinning of both the choroidal middle vessel layer and the choriocapillaris.51 These disorders are considered to represent different stages of a single disease process. Specifically, PPE is regarded as a precursor to CSC; pachychoroid neovasculopathy may develop secondary to CSC and PPE; and polypoidal choroidal vasculopathy is considered the final manifestation of this disease continuum.52–54
Medication-induced CSC
Glucocorticoids are among the most frequently implicated pharmacological agents in the development of CSC. Their role may involve elevating cyclic adenosine monophosphate levels within RPE cells, leading to dysfunction of ion transport mechanisms and increased permeability of the blood-aqueous barrier. These alterations ultimately compromise the integrity of the outer blood-retinal barrier, facilitating SRF accumulation. Notably, CSC patients exhibit significant sympathetic nervous system hyperactivity coupled with reduced parasympathetic tone, compared to healthy controls. This autonomic imbalance underscores the clinical association between CSC and sympathomimetic agents, such as pseudoephedrine, oxymetazoline, ephedra (commonly found in bodybuilding supplements and weight-loss products), and the illicit amphetamine derivative 3-methoxy-4,5-methylenedioxyamphetamine.55 Furthermore, emerging evidence suggests that the use of phosphodiesterase-5 inhibitors, such as sildenafil, may contribute to CSC onset, potentially through nitric oxide-mediated choroidal vasodilation. Intriguingly, the atypical antipsychotic quetiapine, which modulates dopaminergic and serotonergic pathways, has also been associated with CSC development.56 This observation suggests a possible mechanistic role for neurotransmitter-mediated regulation of choroidal vascular permeability in CSC pathogenesis. Collectively, these pharmacological associations highlight the multifactorial interplay between neuroendocrine signaling, vascular dynamics, and RPE dysfunction in CSC.57
Imaging diagnosis
FFA
FFA and indocyanine green angiography (ICGA) are considered the gold standards for diagnosing CSC. FFA typically reveals a single leakage point resembling an inkblot or spray-like leakage). Two leakage points are less common, while multiple points are rare.58 Chronic, subacute, or recurrent cases may display window defects in the RPE with intense fluorescence and very slow or negligible leakage during angiography.59 Although FFA cannot provide detailed images of choroidal circulation, its combination with ICGA is crucial for observing choroidal abnormalities associated with CSC. ICGA can identify delayed filling or hyperpermeability of choroidal capillaries corresponding to areas of RPE leakage, suggesting factors such as choroidal vasospasm or occlusion. These may lead to compensatory expansion of surrounding choroidal capillaries. During mid-phase angiography, increased choroidal permeability becomes evident in the inner layers of the choroid, while late-phase angiography reveals a characteristic pattern of choroidal hyperfluorescence, often accompanied by shadowing from larger choroidal vessels.1
ICGA
ICGA is extensively employed in the diagnosis and management of CSC and to differentiate choroidal neovascularization (CNV) associated with CSC. A hallmark feature of CSC observed in the early phase of ICGA is the presence of well-demarcated hyperfluorescent regions corresponding to dilated choroidal vessels.60 These regions are typically aligned with areas of RPE atrophy or detachment, as visualized by optical coherence tomography (OCT). In the intermediate phase of ICGA, the increased permeability of these dilated vessels results in blurred edges of the hyperfluorescent zones, obscuring the precise location of choroidal vascular dilation. During the late phase, the intermediate hyperfluorescent areas transform, manifesting as continuous hyperfluorescence, a washout-like pattern, or eccentric migration, ultimately forming a hyperfluorescent ring. These areas on ICGA indicate regions with altered autofluorescence. Additionally, hypofluorescence, caused by delayed filling of choroidal arteries and capillaries, can be observed and may persist into the mid-to-late phases of angiography. On ICGA, areas of RPE atrophy appear as hypofluorescent zones, distinguishable around 10 minutes after the start of the angiogram and becoming more pronounced in later stages. This hypofluorescence is thought to result from reduced choroidal capillary perfusion. Compared to FFA, the characteristic “smokestack” leakage pattern in acute CSC appears later and occupies a smaller area on ICGA.61
Recently, ultra-widefield ICGA has been used to observe the extension of dilated choroidal vessels towards one or more vortex vein ampullae before reaching the scleral boundary, suggesting potential vortex vein outflow obstruction .46 Some studies have identified that in pachychoroid spectrum diseases, affected vortex veins demonstrate dilation and leakage, draining into expanded ampullae.62 This indicates that the dilated choroidal vessels observed on ICGA may represent branches of the vortex veins, and obstruction occurring as the vortex veins traverse the sclera leads to vortex vein stasis. Asymmetric dilation and outflow obstruction of the vortex veins may increase the permeability of macular choroidal capillaries, serving as a potential triggering factor for CSC.
OCT
OCT is a critical modality for diagnosing and assessing CSC.63 This non-invasive, contact-free imaging technique provides high-resolution cross-sectional images of the retina, facilitating detailed observation of changes in retinal layer structures. In patients with CSC, OCT distinctly depicts the accumulation of SRF, which typically occurs between the neurosensory retina and the RPE.1 OCT imaging allows for the evaluation of the extent and severity of retinal detachment and assists in quantifying SRF volume.64 Additionally, OCT is useful in detecting abnormalities in the RPE, such as localized elevations or defects , which may be associated with pathological choroidal vascular permeability.65
Repeated OCT assessments enable clinicians to dynamically monitor changes in the disease and the outcome of therapeutic interventions.61 Following treatment, reductions in SRF and the restoration of retinal layers can be visually assessed through OCT, providing a basis for adjustments to the treatment plan.66 OCT not only plays a vital role in the timely diagnosis and assessment of CSC but is also invaluable in long-term follow-up, offering reliable imaging evidence for evaluating disease progression and therapeutic efficacy.67
Optical coherence tomography angiography (OCTA)
OCTA is an innovative imaging technology that evaluates the vascular structure and hemodynamics of CSC without the need for contrast agents. It provides high-resolution images of retinal and choroidal microvascular structures and their dynamics.68 In CSC patients, OCTA can reveal abnormalities in the choroidal vasculature, such as capillary dilation and other vascular changes associated with the condition.69 Bonini Filho et al.70 found that the OCTA device demonstrated a sensitivity and specificity of 100% in detecting CNV in eyes with chronic CSC, showing a high degree of concordance with the gold standard of FFA. Furthermore, OCTA enables clinicians to observe sub-RPE blood flow changes and distinguish minute subretinal neovascularization, which, although uncommon in CSC, can significantly influence prognosis and treatment strategy.71 A prospective study indicated that when dye angiography is not available, OCTA combined with structural OCT assessment can serve as the preferred initial examination for CNV screening in CSC patients. However, there are important considerations when interpreting CNV in CSC eyes on OCTA, such as extrafoveal, small lesions, and RPE undulations due to microrips.72
Additionally, OCTA is useful for evaluating and monitoring treatment efficacy.73 By comparing pre- and post-treatment OCTA images, alterations in retinal and choroidal blood flow can be assessed, aiding in determining whether treatment has effectively improved pathological vascular abnormalities.74 Research by Wu et al.75 demonstrated that OCTA reveals high rates of CNV after photodynamic therapy (PDT) in chronic CSC patients, suggesting that OCTA may serve as the primary approach for CNV identification in this patient population. The non-invasive, high-resolution nature of OCTA makes it a valuable tool in diagnosing and managing CSC over the long term, enhancing clinicians’ understanding of CSC’s underlying pathophysiology and progression.76
However, compared to FFA, OCTA may not effectively identify points of RPE leakage, and only a minority of OCTA findings exhibit typical CSC characteristics. In cases where serous retinal detachment exceeds 485 µm, OCTA images may be affected by artifacts that impair image quality. As such, OCTA still lacks the capacity to replace FFA in CSC diagnosis. Nevertheless, given its non-invasive and straightforward nature, OCTA is advantageous for follow-up examinations, serving as a non-harmful method to evaluate CSC activity and potentially advancing research into its pathogenic mechanisms.
Treatment
Observation
In the majority of cases, CSC resolves spontaneously within several months, leading to favorable recovery of vision. However, there remains a potential risk for permanent vision loss. For newly onset acute serous macular detachment, a period of observation is generally recommended for the first three months. During this time, it is crucial to eliminate precipitating factors and ensure adequate rest.2
PDT
PDT was initially developed for the treatment of CNV. Subsequent observations of choroidal hypoperfusion in areas treated with PDT have provided a rationale for its application in treating CSC.77 PDT not only selectively occludes CNV but also affects the endothelial cells of the choroidal capillaries. When administered at a clinical dose, PDT can transiently and selectively close choroidal capillaries without causing damage to the RPE or the neurosensory retina.78 The therapeutic effects of PDT are achieved through several mechanisms: (1) direct cytotoxicity to tissue cells mediated by phototoxic effects; (2) acute damage to the microvasculature, leading to local ischemia and subsequent secondary cell death; and (3) activation of the local immune system, resulting in the production of numerous complement proteins and cytokines that contribute to the response.
In the context of CSC, PDT facilitates the closure of leaking choroidal capillaries, reducing choroidal blood flow and preventing the accumulation of SRF. Traditional full-dose PDT uses a standard dose of verteporfin (6 mg/m2), with a light dose rate set at 600 mW/cm2 over 83 seconds and a total light energy of 50 J/cm2. Although PDT has demonstrated good efficacy in eliminating SRF, it is not entirely free from risks to ocular structures. Studies have shown that the cytotoxicity and vascular damage associated with PDT are dose-dependent. As a result, several modified PDT protocols have been proposed to reduce treatment-related complications, including half-dose PDT, half-dose-half-fluence PDT, and half-time PDT. The specific parameters of these modified PDT protocols and the related research results are presented in Table 1.78–82
Comparison of photodynamic therapy protocols for central serous chorioretinopathy
| Protocol | Verteporfin dose (mg/m2) | Light energy (J/cm2) | Light dose rate (mW/cm2) | Laser duration (seconds) | Reported study | Study design | Number of eyes | Complete resolution of subretinal fluid (%) at final follow-up | Changes in BCVA |
|---|---|---|---|---|---|---|---|---|---|
| Standard full-dose PDT | 6 | 50 | 600 | 83 | Funatsu et al., 202378 | Retrospective study | 22 | 81.8% (at 3 months after treatment) | Not reported |
| Half-dose PDT | 3 | 50 | 600 | 83 | Fujita et al., 201579 | Retrospective study | 204 | 89.2% (at 12 months after treatment) | Mean LogMAR BCVA improved from 0.11 ± 0.25 before to −0.01 ± 0.22 at 12 months |
| One-third-dose PDT | 2 | 50 | 600 | 83 | Farvardin et al., 202581 | Retrospective study | 72 | 71.4% (at 12 months after treatment) | Mean BCVA increases from 72.4 ± 3.9 to 77.1 ± 5.6 letters |
| Half-dose-half-fluence PDT | 3 | 25 | 300 | 83 | Park et al., 201980 | Retrospective study | 43 | Not reported | Not significantly improved |
| Half-time PDT | 6 | 50 | 600 | 42 | Sheptulin et al., 201882 | Retrospective study | 114 | 87% (at 12 months after treatment) | Median LogMAR BCVA improved from 0.22 before to 0.1 at last visit |
The efficacy differences among these various approaches have been compared in numerous studies. Fujita et al.79 demonstrated that half-dose PDT is effective in treating chronic CSC with relatively fewer complications. Park et al.80 compared the effects of full-dose, half-dose, and half-dose-half-fluence PDT on chronic CSC, finding that both full-dose and half-dose PDT significantly improved visual acuity and reduced SRF, while the effect of half-dose-half-fluence PDT was comparatively weaker. Farvardin et al.81 compared half-dose and one-third-dose PDT in patients with chronic CSC, revealing that both were effective in improving visual and anatomical outcomes. However, half-dose PDT was associated with a higher rate of SRF resolution, greater visual gains, and lower recurrence rates compared to one-third-dose PDT. Baseline factors such as central retinal thickness and leakage patterns on fluorescein angiography significantly influenced treatment outcomes, underscoring the importance of individualized therapy plans.81 Sheptulin et al.82 found that half-time PDT is a safe and effective treatment option for chronic CSC patients, with significant improvements in best-corrected visual acuity (BCVA) during follow-up. A multicenter retrospective study comparing half-dose and half-time PDT for treating CSC showed that both protocols were effective and safe, demonstrating similar efficacy in visual improvement and SRF resolution.83 The studies above confirm the effectiveness of various modified PDT protocols for CSC, with half-dose PDT showing particularly robust efficacy. Future research should focus on larger-scale prospective studies to further optimize dosing strategies and enhance treatment outcomes.
PDT has been recognized as an effective method for treating CSC and was once considered a first-line therapy for this condition. However, its widespread implementation has been limited in recent years due to limitations in drug availability.
Subthreshold diode micropulse (SDM) laser
SDM laser photocoagulation is a high-frequency, brief, subthreshold, and selectively photocoagulative technique that divides a continuous laser beam into shorter bursts. This method minimizes thermal accumulation due to its low energy and minor thermal stacking effects, reducing collateral damage to adjacent tissues. Notably, the 577 nm yellow laser utilized in SDM is less likely to be absorbed by macular xanthophyll, which mitigates photoreceptor damage, making the treatment safer. SDM is favored by many clinicians due to its safety and minimal invasiveness, and it has become the preferred treatment for patients with leakage sites located within the avascular zone of the macula.84
Currently, there is no standardized protocol for SDM treatment parameters or location for CSC, as these vary depending on the lesion site and the treating physician.85 Both 810 nm near-infrared light and 577 nm yellow light are commonly used, with the latter often preferred for lesions at the fovea. Different researchers have adopted varying treatment approaches. Treatment locations can be categorized into three main types: Targeting areas of increased choroidal vascular permeability to reduce leakage, typically using ICGA to identify hyperfluorescent regions in the choroid. Targeting damaged RPE cells to restore their barrier and pump functions, utilizing FFA to identify active leakage sites. Expanding the second category by including adjacent normal retina, and potentially treating the fovea to reinforce barrier functions and offer both therapeutic and preventive benefits. This can include applying photocoagulation to a disc-diameter area centered on the leakage point or utilizing OCT to identify serous retinal detachment areas.86
In a randomized controlled trial involving patients with acute CSC, SDM laser treatment significantly improved BCVA and contrast sensitivity compared to observation alone. Additionally, it reduced recurrence rates of neurosensory detachment without any adverse effects, suggesting that SDM laser is a superior therapeutic option for managing acute CSC.84 Another retrospective case series indicates that subthreshold MicroPulse diode laser treatment may effectively reduce macular thickness and improve visual outcomes in patients with symptomatic chronic CSC, demonstrating its potential as a treatment option for this condition.86 Furthermore, a randomized controlled trial shows that both 532 nm and 810 nm subthreshold micropulse lasers provide comparable efficacy and safety in improving BCVA and resolving SRF over six months in patients with non-resolving CSC, with no observed adverse effects from either laser treatment.87
Laser photocoagulation
Laser photocoagulation therapy is currently regarded as one of the most effective methods for treating CSC, with minimal complications.88 This treatment involves using a laser to coagulate the leakage points in the RPE, thereby sealing RPE defects, enhancing the healing response of damaged RPE, and stimulating healthy RPE cells to participate in tissue repair.89 Alternatively, it can directly activate the pump function of RPE cells adjacent to the leakage area, promoting the absorption of SRF.
Currently, there is no unified and widely recommended standard protocol for energy parameter settings in laser treatment for CSC. Treatment plans are typically adjusted based on individual patient needs by the physician. Maltsev et al.90 adjusted the laser power in the extrafoveal region to achieve minimal visible retinal damage as the treatment endpoint. Ambiya et al.91 used a 577 nm yellow laser, titrated to produce a barely visible burn (mild retinal whitening effect) outside the vascular arcade, with a continuous wave laser having a test spot size of 100 µm and an exposure time of 0.1 seconds.
Regarding the effectiveness of laser treatment, Hara et al.92 demonstrated that focal laser therapy can significantly reduce the volume of choroidal vessels and stroma, with efficacy comparable to PDT. Research by Maltsev et al.93 indicated that in patients with CSC complicated by secondary CNV, complete resolution of SRF was achieved within 1.1 ± 0.4 months post-laser treatment, with follow-up at 11.5 ± 7.5 months showing no deterioration in anatomy or vision. Compared to half-dose PDT, focal laser photocoagulation demonstrates comparable anatomical and functional recovery during follow-up periods of three to 36 months. However, during the three-year follow-up, focal laser photocoagulation exhibited a higher recurrence rate.94,95 Therefore, while focal laser photocoagulation shows definitive efficacy in terms of anatomical and functional recovery, it may be associated with a higher recurrence rate in long-term follow-up. This could be attributed to the fact that CSC is primarily caused by choroidal capillary dilation and leakage, which focal laser photocoagulation does not adequately address. Furthermore, laser treatment is unsuitable for leakage points located beneath the foveal center or within the avascular zone of the macula due to the destructive nature of its mechanism.
Anti-vascular endothelial growth factor therapy (VEGF)
In clinical practice, anti-VEGF agents, such as ranibizumab and bevacizumab, are frequently administered via intravitreal injections. These drugs function by reducing choroidal capillary permeability and limiting neovascularization, thereby decreasing SRF.96 This approach is commonly used for treating CNV secondary to age-related macular degeneration. Clinical trials related to CSC suggest that anti-VEGF therapy may be beneficial for patients with prolonged disease duration and CNV resulting from RPE decompensation, particularly in those concurrently experiencing Type 1 CNV.97 However, robust evidence supporting the efficacy of this treatment in CSC associated with CNV remains limited. For patients who are averse to frequent intravitreal injections, a combination of anti-VEGF therapy and PDT may be considered. Overall, the therapeutic potential of anti-VEGF agents may be confined to CSC cases with concurrent CNV, and their efficacy still requires further validation.98 Currently, anti-VEGF medications are not established as a first-line treatment modality for CSC.99 Continued exploration through pathophysiological studies, foundational experiments, and clinical trials is essential to better understand their role and effectiveness in this context.100
Oral mineralocorticoid receptor antagonists (MRAs)
Research has demonstrated that both endogenous and exogenous corticosteroids can bind not only to glucocorticoid receptors but also to mineralocorticoid receptors (MRs).101 Excessive stimulation of MR can lead to vasodilation and increased osmotic pressure, resulting in the accumulation of SRF. MRAs can inhibit the binding of glucocorticoids or mineralocorticoids to MR, thereby suppressing vasodilation. Overactivation of the MR pathway is considered one of the pathological mechanisms in the development of CSC.102
The primary MRAs used clinically are spironolactone (a diuretic) and eplerenone (an antihypertensive agent).103 Oral spironolactone has been shown to yield significant improvements in central macular thickness, SRF height, and subfoveal choroidal thickness in patients with CSC, although visual acuity remains unchanged. Recurrence is notably higher, especially in older patients and those with prior bevacizumab treatments, although no permanent adverse effects have been reported.103 Spironolactone significantly reduces SRF and subfoveal choroidal thickness in patients with nonresolving CSC, with no observed impact on BCVA or treatment-related complications.104 Eplerenone has been found to be safe but not significantly more effective than placebo in enhancing BCVA for patients with chronic CSC over a 12-month period, suggesting the need for further exploration of alternative treatments for this challenging condition. Furthermore, trials have indicated that oral eplerenone is effective for chronic CSC, resulting in significant reductions in central SRF height, central macular thickness, and subfoveal choroidal thickness, along with a notable improvement in mean BCVA.105 In summary, oral MRAs, particularly eplerenone, appear to be effective for chronic persistent and recurrent CSC. Nonetheless, further randomized controlled trials are required to confirm their efficacy and explore optimal dosing and administration strategies. The oral administration route offers the advantage of avoiding potential tissue damage associated with other treatment modalities, suggesting that low-dose oral MRAs may become a promising alternative for the treatment of persistent and recurrent CSC in the future.
This review has several limitations. Firstly, although some research has been conducted on the potential mechanisms of CSC, the underlying complexities are not fully understood, and the relative contributions of various risk factors, such as psychological stress, hormonal influences, and genetic susceptibility, remain contentious. Secondly, despite significant advancements in imaging techniques, such as OCT and OCTA, data regarding the long-term efficacy and safety of emerging therapeutic approaches are still limited. Lastly, this review does not delve deeply into the psychological and psychosocial factors associated with CSC, which may significantly impact patient management and treatment outcomes. Therefore, future research needs to address these limitations and further elucidate the multifactorial nature of CSC to aid in the development of more targeted therapeutic strategies.
Conclusions
CSC is characterized by choroidal vasodilation resulting from dysregulation of the CRH and glucocorticoid axes under stress conditions, leading to disruption of the RPE tight junctions. PDT was once the first-line treatment option, while other therapeutic approaches continue to evolve. With advancements in imaging and genetic testing technologies, our understanding of the pathogenesis of CSC has significantly deepened. Oral medications and gene therapy may emerge as potent preventive and therapeutic methods, while laser photocoagulation and intravitreal injections may effectively alleviate local symptoms. With the rapid emergence of novel technologies, there is optimism that CSC may soon be effectively and promptly treated.
Declarations
Acknowledgement
None.
Funding
This research was supported by the National Natural Science Foundation of China (81800850).
Conflict of interest
The authors declare no conflict of interest.
Authors’ contributions
Conceptualization, writing - review & editing the manuscript, visualization (EJ), and writing - editing the manuscript (YZ, WL, YC). All authors made significant contributions to this study and approved the final manuscript.

 Author information
Author information