Introduction
Parkinson’s disease (PD) is classically diagnosed by movement disorders, including bradykinesia, rigidity, tremor, and postural instability.1 Eventually, motor symptoms such as tremors, rigidity, slow movement, depression, and dementia become evident.2 Biochemically, these symptoms result from dopamine (DA) depletion in the projections from the substantia nigra pars compacta to the striatum,3,4 particularly in the nigrostriatal pathways. This depletion is probably due to aging, brain injury, toxicity, or other factors; hence, PD is still considered an idiopathic disorder. Several mechanisms have been implicated in PD pathogenesis, with α-synuclein aggregation being central to disease development. In familial cases of PD, many genes are involved, especially as abnormal intra-neuronal aggregates of α-synuclein, also called Lewy bodies, are found in the PD brain.5,6 Multiple other processes are thought to contribute, with several studies suggesting that abnormal protein clearance, mitochondrial dysfunction, and neuroinflammation play roles in PD onset and progression. However, the relationships between these pathways remain unclear.7 In brief, genetic susceptibility and environmental factors,7 which may cause endoplasmic reticulum stress,8 mitochondrial dysfunction, inflammation, and disruption of the autophagy system,9 contribute to PD development.
Currently, there is no cure for PD. Levodopa was introduced in the 1960s to replenish DA loss, but it serves only as a palliative treatment. Furthermore, chronic use of levodopa may result in significant adverse effects such as nigrostriatal degeneration and dyskinesia.10 Other therapeutic approaches—including cell therapy, gene therapy, and deep brain stimulation—are still in the trial stages.
In this context, nutritional management offers a promising option to help control disease symptoms, as foods contain antioxidants, trace metals, and energy that may help counteract cognitive decline and muscle weakness. Epidemiological and biochemical studies suggest that the right choice of foods can help manage PD symptoms.11–20 Recent studies have revealed that certain nutrients may reduce the risk of PD, while others may contribute to neurodegeneration or exacerbate disease progression.21 This review summarizes studies addressing these issues and describes in detail the nutrients and their putative mechanisms of action in PD. In particular, we will focus on the types of foods that promote or slow PD symptoms.
The effects of macro-nutrients on PD symptoms
Carbohydrates
Carbohydrates can allow the DA precursor, the amino acid tyrosine, to cross the blood-brain barrier into the cerebrospinal fluid and increase DA production there.22,23 In fact, carbohydrates indirectly facilitate the passage of tyrosine through the blood-brain barrier by influencing the ratio of amino acids in the blood. High-carbohydrate meals increase the plasma tyrosine-to-large neutral amino acids ratio, promoting tyrosine uptake into the brain.24,25 Carbohydrate-rich foods that are rapidly broken down and absorbed into the bloodstream are categorized as high-glycemic index (GI) foods. High-GI foods lead to a rapid increase in blood glucose and insulin levels following ingestion. In contrast, low-GI foods are digested more slowly, resulting in a smaller and slower postprandial blood glucose and insulin response. High-GI carbohydrates can cause a rapid increase in blood sugar levels and trigger insulin release from the pancreas. This insulin, in turn, stimulates DA release in the brain. Since PD is characterized by a lack of DA, this mechanism might provide temporary compensation.26
Furthermore, carbohydrates with a high glycemic index may decrease the risk of PD by increasing DA production in the brain through insulin release.26 However, there is also a risk that high-carbohydrate diets may increase the incidence of type 2 diabetes mellitus,27–29 which may increase the risk of PD with severe motor symptoms.30–34
Fat
In animal studies, it was shown that a high-fat diet can deplete DA levels in the substantia nigra and aggravate Parkinson’s symptoms.35–37 Epidemiological studies in humans have demonstrated that a higher number of PD cases occur among individuals who consume large amounts of total animal fat.38–41 In one experiment, a higher-fat diet (Western diet ) and a control diet (standard lab chow) were compared for their effects on the mesolimbic DA system. Twenty male C57BL/6J mice were placed on one of these diets at seven weeks of age. After twelve weeks, in vivo fixed potential amperometry was used to measure real-time stimulation-evoked DA release in the nucleus accumbens of anesthetized mice before and after intraperitoneal injection of the dopamine transporter (DAT) inhibitor nomifensine.
Results indicated that diet altered mesolimbic DA function: mice that consumed the Western diet demonstrated a hypodopaminergic profile, specifically reduced baseline DA release and an attenuated dopaminergic response to DAT inhibition compared to the control diet group. Thus, diet may play a role in mediating DA-related behavior, disorders associated with DA dysfunction, and pharmacological treatments aimed at altering DA transmission.42,43
Researchers studying the effects of high-fat diets (HFDs) on DA levels in rodents often use a 60% fat diet for two weeks to observe changes compared to a control group on a lower-fat diet. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated macaques, six weeks of oral docosahexaenoic acid (DHA, 30 mg/kg/day) reduced levodopa-induced dyskinesia by 40%, as measured by the Parkinsonian scale developed at Laval University.44 This type of study is used to investigate how diet impacts the DA reward system and its relation to behavioral changes. One study aimed to examine early changes in behavior and brain dopaminergic function in young mice fed a HFD. Results showed that initial signs of weight gain and behavioral deficits started in the third month of HFD intake. By the end of the fifth month, the mice fed the HFD had increased DA and dopamine receptor D2 levels in the midbrain, while DAT levels remained unchanged. This research contributes to the fields of nutrition and neuroscience, as identifying early behavioral deficits may reflect initial dysregulation of dopaminergic or other neurochemical pathways, which is critical for understanding the progression of brain disorders induced by HFD.45
Previously, it was found that one-month-old C57BL/6J mice fed an HFD gained significantly more body weight after four months than mice fed the control diet, despite no difference in total food consumption between the groups. This increase in body weight is consistent with other reports. For example, a study using young adult C57BL/6J mice (2 months old) showed significant weight gain after two months on an HFD.46
Another study found that HFD caused young C57BL/6J mice to weigh 12.4% more than controls after six weeks, even though the HFD group consumed less food per day.47 Finally, a study using male Wistar rats aged 14–16 weeks also found significant differences in body weight after four weeks of HFD exposure.48
These results indicate that rodent models from different age groups chronically fed an HFD display greater body weight compared to animals fed control diets. The rate of weight gain on any diet is always a function of age; for example, adult animals might gain weight more quickly due to their slower metabolic rate compared to younger animals.49 Identifying the initial time point for body weight gain and motor behavioral deficits is essential for understanding the impact of HFD in C57BL/6J mice. Such knowledge may help reveal underlying neurophysiological and behavioral conditions that depend on caloric intake. For example, calorically restricted C57BL/6J mice have shown improved cardiometabolic health, hippocampal RNA expression, nutrient-sensing pathways, age-dependent cognitive function, and dendritic spine density compared to mice fed a control diet.50
In contrast, HFD-fed mice showed weight gain, impaired glucose tolerance, deficits in hippocampal-dependent memory and learning, mood disturbances, and depression-like behavior.47 Such results indicate that caloric intake and the time course of weight gain play crucial roles in the etiology of normal brain function (or dysfunction).48
However, a ketogenic diet, which is also high in fat, has shown some disease-modifying activity in PD.46 Yet, some studies have reported no significant differences in Unified Parkinson’s Disease Rating Scale scores or motor function after 28 days of a ketogenic diet compared to low-fat diets. Other research suggests that the ketogenic diet might not be superior to other dietary approaches in terms of physical performance.49 Therefore, although the biological mechanisms linking ketosis and PD are promising, existing evidence from clinical trials is not conclusive enough to recommend it as a standard treatment.50
This may be partly due to the lower protein content (only ∼8%) in the ketogenic diet, which improves the bioavailability of levodopa, a precursor of DA. Mounting evidence shows that saturated fat impacts DA neurons and their terminal fields, but little is known about the effect of a diet high in unsaturated fat on the DA system. However, Barnes et al.51 showed that a diet high in unsaturated fat may preserve normal metabolic and behavioral parameters as well as DA signaling in the nucleus accumbens.
Polyunsaturated fatty acids and monounsaturated fatty acids can reduce the risk of PD.52,53 Polyunsaturated fatty acids exhibit anti-inflammatory and neuroprotective properties.54–57 while monounsaturated fatty acids can reduce oxidative stress.58,59 Furthermore, α-linolenic acid, an essential fatty acid, can protect brain cells from oxidative stress and inflammation,60–62 thereby benefiting PD patients.63
High levels of cholesterol, which is required for cell membranes, have been found to be associated with decreased PD symptoms, but only in women, not in men.53 More recently, the role of fat in PD is thought to depend on the type of fat. For example, the high-density lipoprotein/low-density lipoprotein ratio in the patient’s diet is inversely related to disease duration and provides cardiometabolic protection in PD.53,64 While higher cholesterol levels might be associated with a lower risk of PD, the exact role of cholesterol in PD pathogenesis is still under investigation. Research suggests that cholesterol plays a crucial role in the structure and function of neuronal cell membranes and synapses.
Some studies have indicated that higher low-density lipoprotein cholesterol levels might be linked to slower progression of motor and executive dysfunction in individuals with PD. However, other studies have shown that cholesterol deficiencies in nerve cells can cause defects in cell membranes, potentially leading to neurodegeneration. In conclusion, while higher cholesterol levels may be associated with a lower risk of PD and some studies suggest a protective role, the exact mechanisms and cholesterol’s role in PD development and progression remain under investigation, with some research showing contradictory results.65
Omega-3
Dietary supplementation with omega-3 fatty acids, including DHA and eicosapentaenoic acid (EPA), has been shown to increase DA levels and D2 receptor binding, and to reduce monoamine oxidase B activity in the prefrontal cortex and D2 receptor binding in the striatum.66,67
DHA can also inhibit nitric oxide (NO) production and calcium influx, thereby reducing apoptosis of dopaminergic cells.68 Additionally, DHA may protect the brain by increasing the activities of glutathione peroxidase and glutathione reductase, both antioxidant enzymes.69 Short-term administration of DHA has been shown to reduce levodopa-induced dyskinesia by 40% in Parkinsonian primates.44 In MPTP-treated macaques, a six-week oral DHA regimen (30 mg/kg/day) reduced levodopa-induced dyskinesia by 40%, as measured using the Parkinsonian scale developed at Laval University.44 EPA is also a neuroprotective agent, as observed in experimental models of PD.70–72 In one study using a head injury model, 40 adult male Sprague-Dawley rats received dietary supplementation with n-3 fatty acids (EPA:DHA = 2:1) at dosages of 10 or 40 mg/kg/day starting on post-injury day one. The authors found that, compared to injured rats on a control diet, n-3 fatty acids significantly reduced the number of beta-amyloid precursor protein-positive (injured) axons at 30 days post-injury, achieving levels similar to those in uninjured animals.73 A diet rich in EPA can reduce hypokinesia in MPTP-induced mouse models and protect against memory decline.73
Effects of proteins
Meat
A positive association between red meat consumption and PD may be due to its heme content, which, when not digested properly, can act as a toxin, producing hydroxyl radicals and causing mitochondrial damage.74 However, the evidence for this association is conflicting.75
Fish
Omega-3s and the calcium-binding protein parvalbumin are abundant in fish muscle tissue, especially in herring, cod, carp, redfish, salmon, and red snapper. These compounds can inhibit the formation of abnormal α-synuclein aggregates, which are associated with the onset of PD.76,77
Several epidemiological studies have revealed that adhering to a diet containing fish oil is associated with a reduced incidence of PD. A follow-up study interviewing 131,368 people about their food intake reported that consuming a diet rich in fish oil was associated with a decreased risk of PD.78 Similarly, another epidemiological study involving over 5,000 patients verified that a diet rich in fish oil is directly associated with a lower risk of PD development.79
In addition, laboratory research showed that fish oil consumption for seven days can restore DA levels in the brains of rats with traumatic brain injury, which is associated with an increased risk of PD.80 Overall, fish is a recommended food for PD patients.81
Eggs
Beneficial nutrients for brain health, such as vitamin D and omega-3 fatty acids, are present in eggs.82 However, the protein content in eggs may interfere with the absorption of levodopa medication if taken together.83
Effects of micronutrients (vitamins and trace metals) on PD: Vitamins D, C, and E
Vitamin D deficiency is prevalent in PD patients,
84 but it is not known whether vitamin D deficiency causes PD. Vitamin D plays a role in regulating calcium homeostasis,85 ,86 and disruption can cause the loss of dopaminergic neurons.87 In animal and cell culture models of PD, vitamin D supplementation was found to slow disease progression.88 ,89 However, in humans, results were opposite, with vitamin D increasing the risk of PD,89 indicating the need for more careful research in this area.Vitamin C (ascorbic acid) is highly effective in reducing lipid peroxidation levels and increasing catalase activity.
90 However, the association between vitamin C and PD risk remains inconclusive, as other studies did not find a significant correlation.91 ,92 Vitamin E, a lipid-soluble vitamin that provides protective effects on DA neurons in the substantia nigra pars compacta and reduces DA loss in in vitro and in vivo experiments.
91 ,92 Pre-treatment with vitamin E reduces lipid peroxidation levels.93 A meta-analysis showed a protective effect of vitamin E against PD in humans94 ; however, clinical trials with PD patients did not show such neuroprotective functions.95 ,96 Trace metals, Iron-induced oxidative stress is a known factor in PD pathogenesis. Clinical trials with iron chelators (such as deferiprone) have yielded mixed results, with some showing no benefit and others indicating potential benefits.
97
Other foods and drinks associated with decreased or increased risk of PD
Fruits and vegetables:Most fruits and vegetables are rich sources of various phytochemicals, including antioxidants and vitamins A, B (riboflavin), C, and E. These compounds can inhibit lipid peroxidation, as well as glutathione peroxidase activity and glutathione levels in the substantia nigra.
98 ,99 In mice, pretreatment with β-carotene partially protects against MPTP-induced neurotoxicity,100 ,101 but this effect is not seen in primates.102 Additionally, low intake of vitamin B6 has been associated with an increased risk of PD.103 Cruciferous vegetables such as cauliflower, cabbage, and broccoli are rich in antioxidants like sulforaphane and erucin, which have significant neuroprotective capacity.104 ,105 Soy (Genistein): The primary soybean isoflavone, genistein, is a source of protein with neuroprotective capacity. These results were found in chemically induced ovariectomized rat models, suggesting that soy may be useful for the prevention of PD in postmenopausal women.
106 Further, genistein can inhibit microglia activation and neuron loss in PD.107 Dairy products: Consumption of dairy products and milk may increase the risk of PD.
108 –111 Additionally, the possible presence of dopaminergic neurotoxins, such as pesticides and polychlorinated biphenyls in dairy products, may increase PD risk.109
Drinks
Caffeine
Epidemiological studies support an inverse relationship between PD and coffee consumption.112–114 Generally, animal studies also indicate that caffeine is neuroprotective. Administration of caffeine to manganese ethylene-bis-dithiocarbamate and paraquat-treated rodents, while normalizing the expression of interleukin-1 beta, p38 alpha mitogen-activated protein kinase, nuclear factor kappa B, and tyrosine kinase, reduced the number of degenerating dopaminergic neurons.115,116 Both acute and chronic administration of caffeine can also reduce the loss of striatal DA in rats treated with MPTP and 6-hydroxydopamine.117,118
Genetic and pharmacological studies in rodents indicate that caffeine can reduce dopaminergic toxicity and slow disease progression through inhibition of adenosine receptor subtype A2A.119–121 Currently, clinical studies are underway to evaluate A2A receptor antagonism for symptomatic relief or slowing PD progression.122 Caffeine can also activate the phosphatidylinositol 3-kinase/protein kinase B signaling pathway, as shown in SH-SY5Y cells.123 Therefore, it is speculated that caffeine might downregulate NO production, neuroinflammation, and microglial activation, contributing to neuroprotection.124
Tea
Several epidemiological studies and experiments with PD animal models have shown that regular tea consumption can protect against the onset of PD.125–127 Polyphenols in tea extracts are potent antioxidants that exhibit radical scavenging activities and provide neuroprotection in cell culture and animal models.128–131 Theaflavins, a group of polyphenols found in both black and oolong tea, possess various pharmacological properties such as antioxidative, anti-apoptotic, and anti-inflammatory effects.132,133 Theaflavin-mediated neuroprotection in MPTP-induced PD animal models was demonstrated by reduced expression of apoptotic markers and increased expression of nigral tyrosine hydroxylase and DAT enzymes.134 Epigallocatechin-3-gallate, present in green tea, has shown the ability to reduce NO production in MPTP mouse models of PD, providing further evidence for its neuroprotective properties.135 In contrast, another study using 6-hydroxydopamine-lesioned rats found only subtle symptomatic relief but no neuroprotection at similar doses of epigallocatechin-3-gallate.136 This discrepancy may be due to differences in the mechanisms by which these chemicals induce PD-like lesions in animals.137
Alcohol
Alcohol is believed to be neuroprotective concerning PD lesions.138 A recent study supports that low to moderate beer consumption lowers the risk of PD; however, alcohol addiction may have the opposite effect.139 Other studies have shown that the main components of alcohol, such as resveratrol and quercetin, are neuroprotective. These compounds can prevent behavioral, biochemical, and histopathological changes in MPTP-induced mouse models.140,141 Biochemically, resveratrol scavenges free radicals, thereby preventing inflammation and apoptosis of dopaminergic neurons.142–144 However, epidemiological studies do not support any benefit from red wine consumption regarding PD symptoms.144
Pro/Con evidence for major dietary approaches (Mediterranean, ketogenic, etc.)
Both the Mediterranean diet and the ketogenic diet show promise for individuals with PD, though their mechanisms and effects differ. The Mediterranean diet, rich in whole grains, fruits, vegetables, and healthy fats, is associated with a reduced risk of PD and may help manage non-motor symptoms like constipation and improve cognitive function. The ketogenic diet, a high-fat, low-carbohydrate diet, focuses on inducing ketosis, potentially improving brain function and alleviating some PD symptoms, including non-motor symptoms. The differences of the activities between the Mediterranean and the Ketogenic diets on PD are portrayed in Table 1.145–150
Differences of Activities of the Mediterranean diet and the Ketogenic diet in relation to PD
| Potential beneficial effects on PD | Mediterranean diet | Ketogenic diet |
|---|---|---|
| Antioxidant effects | Yes | No |
| Specific nutrients and PD risk: Iron: potential pro-oxidant effects; Vitamins K and C: antioxidant properties. Potential influence on dopamine metabolism;145 Neuroprotection via polyphenols. Improved gut microbiome composition146–148 | Yes | No |
| Enhanced mitochondrial function. Neuroprotection via ketone bodies | No | Yes |
| Potential anti-inflammatory effects | No | Yes |
| May improve both motor and non-motor symptoms. Particularly beneficial for non-motor symptoms | No | Yes |
| Potential for metabolic health149,150 | No | Yes |
Brain–gut–microbiome interactions and intermittent fasting (IF) in PD symptoms
IF can alter the gut microbiome, potentially influencing PD symptoms. IF may shift the balance of gut bacteria, increasing diversity and promoting beneficial microbes. Sex-specific nutritional responses also play a role, with some studies suggesting that men may be more responsive to IF-induced improvements in cardiometabolic health compared to women. The gut microbiome is known to interact with the brain through the gut-brain axis, and alterations in the gut microbiome could potentially influence PD development and progression.
Changes in the gut microbiome could impact various aspects of PD, including motor and non-motor symptoms.
151 Some research indicates that the effects of polyphenols on gut microbiota and metabolic health may be sex-specific, with men showing greater responsiveness to certain polyphenols.
Animal studies suggest that IF can protect neurons, improve motor function, and reduce alpha-synuclein burden in the brainstem, a key area affected in Parkinson’s.
Some studies also suggest IF may have neuroprotective effects by promoting autophagy (cellular cleanup) and reducing oxidative stress.
152 More research is needed to explore the specific effects of IF on the gut microbiome in individuals with PD and to determine whether IF can be used as a therapeutic strategy for managing PD symptoms.
Translational considerations
This subsection discusses practical dietary recommendations for clinicians and patient adherence challenges. Clinicians can provide practical dietary recommendations focused on overall health, including a balanced diet rich in fruits, vegetables, whole grains, and lean protein, while minimizing saturated fat, added sugars, and sodium. Adherence challenges include lack of motivation, time constraints, and affordability of healthy food options, alongside the influence of marketing and social factors. Strategies to improve adherence include education, personalized guidance, and social support.153
Practical dietary recommendations
Focus on a balanced diet that includes higher-fiber starchy carbohydrates, lean proteins, and healthy fats, along with at least five servings of fruits and vegetables daily.154 Whole grain products are recommended over refined grains for better nutrition and to avoid vitamin deficiencies.155
Limit saturated fats, sugars, and sodium (<2.3 g per day) to promote cardiovascular health.156 Trans-fats should be completely avoided. Additionally, portion control, rather than overeating, is important for maintaining good health.157 Address social and cultural issues wisely so they do not abruptly influence your dietary choices.158 Along with these dietary recommendations, regular leisure-time physical exercise, at least one hour daily, is highly recommended to maximize nutritional benefits.
Clinical translation challenges, gaps in current evidence, and priority directions for future research
PD research has progressed enormously in recent years, unlocking the mysteries of Parkinson’s and making treatments that restore lost function, halt disease progression, and prevent the condition more realistic goals. Several genetic mutations that increase susceptibility to PD have been identified, and breakthroughs in genetic research have made finding new genetic factors easier and more efficient. A number of promising new therapies have been developed and are currently being tested in animals as well as humans. Additionally, research into the underlying biology of the disease, environmental influences, and new biomarkers is ongoing. There is hope that new therapies will continue to improve symptom relief, reverse progression, or even prevent the occurrence of PD.159,160
Advances in neural circuitry research have also accelerated rapidly in recent years. A wide spectrum of tools and techniques can now map connections between neural circuits. Using animal models, scientists have shown how neural circuits in the brain of zebrafish work precisely in behavioral responses such as seeking and capturing food. Together, these studies may yield tools and technologies that deepen our understanding of how the nervous system functions in health and disease, including PD.161,162
Patient adherence challenges
Lack of motivation: Patients may struggle to prioritize healthy eating when it conflicts with other priorities or habits.
163 Time constraints: Busy schedules can make it difficult to plan and prepare healthy meals.
164 Cost of healthy food: The price of healthy foods can be a barrier for some individuals, especially those with low incomes.
165 Marketing of unhealthy foods: Aggressive marketing of unhealthy foods can influence food choices.
164 Food insecurity: Lack of access to affordable and nutritious food can limit healthy eating.
164 Social factors: Family eating habits, cultural norms, and social gatherings can impact dietary choices.
164 Lack of information: Some individuals may lack knowledge about healthy eating principles.
164 Difficulty adjusting habits: Changing established eating patterns can be challenging.
165
Strategies for enhancing adherence
Education: General strategies include education, motivation, behavioral skills training, use of newly available modified foods on the market, and interpersonal interactions.
166 Provide clear and concise information about healthy eating, including its benefits and how to make changes.
167 Personalized guidance: Offer tailored recommendations and support based on individual needs and circumstances.
166 Energy to overcome cognitive decline: This study suggests that individuals with worse cognitive function may choose to eat “neuroprotective components” earlier in the day, when cognitive performance is better. A meal pattern characterized by high energy consumption in the morning or low energy intake at the end of the day could be a marker of cognitive impairment.
Motivational counseling: Help patients identify their own reasons for wanting to make dietary changes and develop strategies for success.
Behavioral skills training: Teach patients skills such as goal setting, self-monitoring, and problem-solving to overcome challenges.
164 Social support: Encourage patients to connect with supportive friends, family, or support groups.
164 Consider cultural and social factors: Acknowledge and address cultural or social influences that may impact dietary choices.
166 Utilize resources: Refer patients to local food banks, community gardens, or other resources that can help them access affordable and nutritious foods.
Address lack of information: Provide education to individuals who may lack knowledge about healthy eating principles.
167
Discussion
In view of the above information regarding the effects of different food components on PD, various dietary patterns can be considered. For example, a protein-restricted diet may help improve the absorption of the PD drug levodopa.168 However, there is a risk of adverse effects due to a shortage of some essential amino acids, which may require protein supplementation.169
A ketogenic diet, which is very low in carbohydrates,170,171 has been shown to potentially alleviate PD symptoms.172 However, the ketogenic diet may cause significant weight loss.173 Therefore, more safety research is needed, especially regarding the relationship between weight loss and cognitive benefits, before recommending long-term adherence to the ketogenic diet.
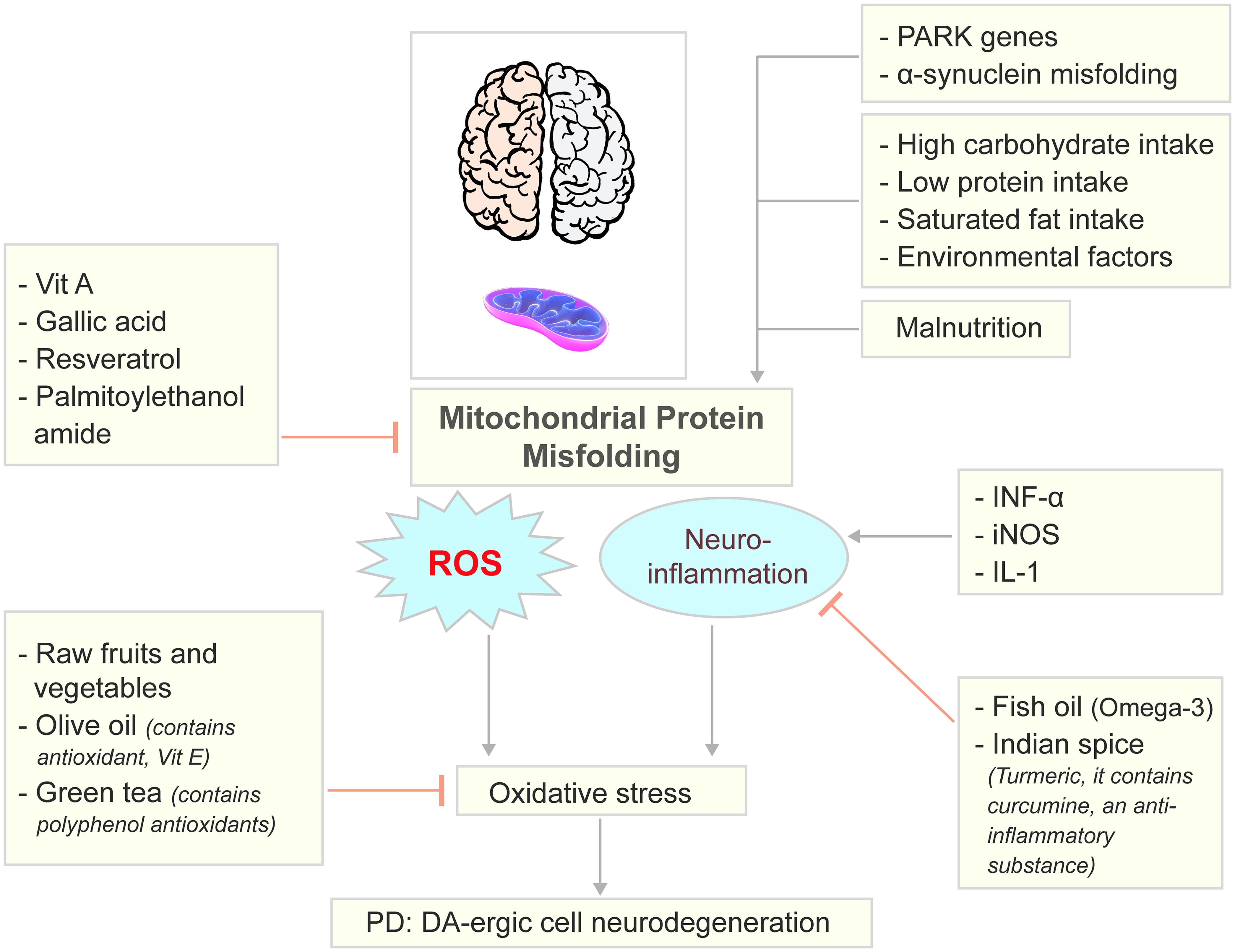
The Mediterranean diet represents a dietary pattern rich in fruits, vegetables, legumes, cereals, nuts, fish, and monounsaturated fatty acids, with moderate alcohol intake but low consumption of dairy products and red meats.174 Previous studies have reported multiple beneficial effects of the Mediterranean diet, including positive impacts on depression,175–177 Alzheimer’s disease,178 and neurodegeneration. However, case–control and cohort studies have yielded mixed results regarding the benefits of the Mediterranean diet in PD prevention and progression.147,179–183 Therefore, further research is needed to better understand the impact of the Mediterranean diet on PD. The combination of the Mediterranean diet and the DASH diet into a single Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay dietary pattern has shown a reduced risk of PD and cognitive decline in both men and women.147,181 Based on these findings, we can illustrate the key nutrient pathways affecting PD pathology and nutritional interventions (Fig. 1).
PARK genes, α−synuclein aggregation as well as malnutrition, low protein intake, and some environmental factors while can cause mitrochondrial protein misfolding and develop PD; Vitamin A, Gallic acid, Resveratrol, and Palmitoylethanolamide blocks the mitochondrial protein misfolding and inhibits the onset of PD. Fish oil, some Indian spice, especially, Turmeric is anti-inflammatory and can inhibit INF-α, iNOS and IL-1 mediated neuro-inflammation and oxidative stress. Likewise, some raw fruits, vegetables, olive oil and green tea can also inhibit oxidative stress. Image elements were adopted from Pixabay.com. DA, dopamine; IL-1, interleukin-1; INF-α, interferon alpha; iNOS, inducible nitric oxide synthase; PARK, parkin; PD, Parkinson’s disease; ROS, reactive oxygen species; Vit A, vitamin A.
Most selected studies include one by Bianchi et al.,183 who investigated the effects of malnutrition and the Mediterranean diet on PD incidence and progression. Other investigations contributed evidence on the critical roles of microbiota, vitamins, polyphenols, dairy products, coffee, and alcohol intake. This review included fifty-two studies that met the inclusion criteria. This study suggests that individuals with worse cognitive function may choose to eat earlier during the day when cognitive performance is better. It is hypothesized that a meal pattern characterized by high energy consumption in the morning or low energy intake at the end of the day could be a marker of cognitive impairment.183
Another study by Ó Breasail et al.184 examined the link between PD and the gastrointestinal tract, based on the Braak hypothesis, although only circumstantial evidence currently exists. The role of empirically observed phenomena such as small bowel gastrointestinal overgrowth is not yet fully understood, particularly regarding its importance in the prodromal phase. The impact of Helicobacter pylori infection on dyskinesia also remains unclear. Adequately powered and well-designed randomized controlled trials are required to assess these links.184
Overall, PD is a neurodegenerative disorder associated with diminished nutritional status and quality of life. Since no preventive or curative therapy currently exists for PD, nutrition and diet represent modifiable risk factors for reducing disease risk. Nevertheless, more research is needed to explore these relationships and the impact of specific diets and dietary patterns.
Conclusions
There are still many concerns regarding the association between PD and nutrition, possibly due to underlying genetic and environmental factors. However, a body of evidence reveals that correcting malnutrition, modulating gut microbiota, and following the Mediterranean diet reduce the onset of PD and slow clinical progression. Other factors, such as polyphenols, polyunsaturated fatty acids, and coffee intake, may have potential protective effects. Conversely, milk and dairy products may increase the risk of PD. Nutritional intervention is essential for neurologists to improve clinical outcomes and reduce disease progression in PD.
In conclusion, dietary interventions for PD management provide promising potential. However, their full integration into personalized medicine, considering timing and individual variation, still depends on rigorous scientific research to clarify their mechanisms of action, efficacy across patient populations, and safety profiles.
Declarations
Acknowledgement
I acknowledge all our staff members and scientists from Sacred Heart University, Chemistry Department, for their support during the writing of this review by providing materials and editing.
Funding
No funding was received.
Conflict of interest
We declare no conflict of interest, financial or otherwise. We also confirm that we have read the journal’s position on issues involved in ethical publications and affirm that this report is consistent with those guidelines. The views expressed in this article are those of the author solely and do not reflect the official policy of the Department of Chemistry or the University.
Authors’ contributions
Information search (AC), writing (AC, SG). Both authors have approved the final version and publication of the manuscript.

 Author information
Author information