Introduction
Muscle degeneration, characterized by structural and functional loss in muscle tissue, is a significant concern following various surgical procedures. In rotator cuff tears, muscle atrophy and fatty infiltration are known predictors of poor surgical outcomes.1 After spinal surgery, back muscle degeneration can occur, with fatty changes being the most common—though not always correlated with pain.2 In cardiomyoplasty, surgical dissection of the latissimus dorsi muscle results in substantial damage and morphological abnormalities, which are further aggravated by chronic stimulation.3 Muscle atrophy occurs when protein degradation exceeds protein synthesis, typically due to factors such as disuse, aging, or systemic disease. In contrast, hypertrophy involves increased protein synthesis and cellular enlargement.4 Understanding these opposing processes is crucial for developing effective treatments and improving surgical outcomes across various medical fields.
Magnesium (Mg) alloys are emerging as promising alternatives to permanent metallic implants in orthopedic applications. Traditional implants—such as titanium, cobalt-chromium alloys, and stainless steel—can cause stress shielding and often require removal surgeries.5,6 In contrast, Mg alloys offer biodegradability, biocompatibility, and mechanical properties that closely match those of human bone.7 During degradation, these alloys promote osteogenesis and angiogenesis, thereby enhancing fracture healing.5 However, challenges such as rapid degradation and hydrogen gas release remain obstacles to clinical application.6 Ongoing research focuses on improving the corrosion resistance of Mg alloys through alloy development, surface treatments, and implant design modifications.8 Despite current limitations, Mg alloys have the potential to reduce long-term complications associated with permanent implants and eliminate the need for removal procedures—making them an attractive option for temporary orthopedic use.7,8 A summary comparison of implant materials discussed in this section is provided in Table 1.9–28
Basic comparison of implant materials in the literature
| Implant material | Advantages | Disadvantages |
|---|---|---|
| Ti alloys | High biocompatibility, mechanical stability, and low corrosion9,10 | Permanent (irreversible), risk of inflammation11,12 |
| Co-Cr alloys | High wear resistance, long service life13,14 | Risk of tissue damage due to high hardness, limited biocompatibility15,16 |
| Stainless steel | Economical, mechanically strong17,18 | High corrosion rate, risk of inflammation19,20 |
| Polymer-based implants | Flexible, biodegradable options available21,22 | Low mechanical strength, limited long-term reliability23,24 |
| Mg alloys | Biodegradable, promotes tissue healing25,26 | Controlled degradation is difficult, and mechanical strength is low27,28 |
Muscle degeneration is a significant concern in orthopedic surgeries, especially those involving implants. In rotator cuff tears, muscle atrophy and fatty infiltration are independent predictors of poor surgical outcomes.1 Similarly, breast implants may lead to sarcopenia and fat degeneration of the pectoralis major muscle, likely due to the weight of the implant and mechanical pressure on muscle fibers.29,30 These mechanical changes can disrupt mechanobiological signaling at the cellular level, affecting muscle architecture, structure, and composition. The clinical implications of muscle degeneration are substantial, influencing both surgical decision-making and rehabilitation strategies. Treatments that benefit atrophic muscle may be harmful to degenerating muscle, and vice versa.31 A deeper understanding of the molecular pathways and mechanical forces involved in muscle degeneration is crucial for developing targeted therapies and optimizing surgical outcomes, particularly in procedures involving implants or muscle repair.
Implant materials can induce a variety of responses in muscle tissue, including inflammation, fibrosis, and mechanical stress. The interaction between an implant and the host tissue is critical for successful integration and may trigger foreign body reactions and immune responses.32 Chronic local inflammation, combined with mechanical factors such as micromotion and stress, significantly influences the long-term behavior of implants.33 Implant-induced changes in intermuscular connectivity can alter force transmission between muscles, potentially impairing skeletal muscle function.34 Additionally, the formation of connective tissue around implants can increase tissue stiffness and alter the configuration of muscle linkages, potentially doubling or quadrupling mechanical interactions within weeks.34 Understanding these complex interactions is crucial for designing new generations of implants that improve biocompatibility and modulate specific tissue responses for repair and regeneration.35
Implant materials also affect surrounding tissues by influencing immune responses, fibrosis, and vascularization. Non-degradable metals like titanium and cobalt-chromium alloys typically cause fibrotic encapsulation.5 In contrast, biodegradable Mg alloys provide distinct advantages. Although Mg degradation may initially increase inflammation, it subsequently promotes immunomodulation and angiogenesis.36 Mg ions released during degradation stimulate both osteogenesis and angiogenesis, making these alloys particularly well-suited for orthopedic applications.5 Muscle tissue can also adapt to the mechanical changes imposed by implants, potentially affecting protein synthesis and cytoskeletal structure.37 Understanding these complex biological responses at the cellular and molecular levels is crucial for predicting long-term outcomes and improving the safety of Mg-based implants.38
Mg alloys have thus gained recognition as promising biodegradable implant materials for orthopedic use, owing to their favorable biocompatibility and bone-like mechanical properties.39 These alloys offer significant advantages over permanent implants, such as eliminating removal surgeries and reducing long-term complications.8 However, their rapid corrosion in physiological conditions has hindered broader clinical adoption.40 To address this, researchers are exploring the use of alloying elements—such as Al, Mn, Ca, Zn, and rare earth elements—to enhance corrosion resistance.40,41 Surface modifications, including polymeric coatings like sol-gel films and synthetic aliphatic polyesters, have also shown promise in improving both corrosion resistance and biocompatibility.40 An ideal biodegradable implant should maintain a balance between gradual material loss and the increasing mechanical strength of newly forming bone tissue.39 Continued research focuses on optimizing alloy composition and surface engineering to control degradation kinetics and enhance overall implant performance.8
The literature reveals a complex interplay between implant materials and surrounding tissues, including skeletal muscle. While bone substitute materials can influence muscle function and adaptation,37 the effects of implants on muscle degeneration remain underexplored. Recent advances in biomaterials for treating volumetric muscle loss are promising, with acellular implants guiding cell fate and tissue organization.42 However, the biodegradation of implant materials in the biological environment remains a major concern.43 Various materials—including metals, ceramics, and polymers—can deteriorate in the body, potentially compromising their mechanical, physical, and chemical properties.44 This degradation process, broadly referred to as corrosion, affects all types of implant materials and can have clinical significance even in cases of seemingly minor reactions. Further research is necessary to understand the long-term impact of implant materials on muscle tissue and to develop more biocompatible and stable solutions.
This review explores the potential of magnesium-based biomaterials for orthopedic implants, highlighting their advantages over traditional metallic implants such as titanium, cobalt-chromium, and stainless steel. Magnesium alloys offer biodegradability—eliminating the need for implant removal surgeries—and possess mechanical properties similar to bone, thereby reducing stress shielding.45 They also demonstrate osteoconductivity and antibacterial properties.46 However, rapid biodegradation and insufficient mechanical strength remain significant challenges.47 To address these issues, researchers have investigated alloying with elements like aluminum and zinc, reinforcing with ceramics, and applying surface coatings.48 The development of Mg-based bulk metallic glasses has also shown promise in improving corrosion resistance.45 Ongoing research focuses on optimizing the balance between degradation rate and mechanical integrity to match bone healing, potentially revolutionizing orthopedic implant materials.46
This review examines the effects of implant materials on muscle tissue and evaluates their associated risks and benefits. Bone substitute materials can influence muscle function by altering fiber type distribution, myosin heavy chain composition, and vascularization.37 In treating volumetric muscle loss, implantable biomaterials with defined structural and biochemical properties can guide cell behavior and tissue formation.42 Non-invasive imaging modalities are crucial for assessing the functional performance of myogenic biomaterials and engineered muscle tissues.49 The foreign body reaction remains a significant concern in biomedical implantation and is influenced by the implant material’s physiochemical properties, which create a protein “fingerprint”.50 A deeper understanding of these interactions is essential for developing implant materials with improved biocompatibility and reduced foreign body reaction, ultimately enhancing outcomes in muscle tissue engineering and regeneration.
Mechanisms of muscle degeneration
Post-implant inflammation and cellular damage
Post-implant inflammation in muscle tissue involves a complex cascade of biological events. Following implantation, proteins adsorb to the biomaterial surface, attracting neutrophils and macrophages.51 This inflammatory response is characterized by rapid immune cell recruitment and cytokine release.52 Neutrophil infiltration occurs early, followed by macrophage accumulation.53 These immune cells recognize damage-associated molecular patterns and produce inflammatory cytokines, which may stimulate muscle repair but can also lead to cytokine storms and further tissue damage.54 The implantation process also triggers angiogenesis, with increased hemoglobin content and vessel formation in the affected muscle.53 Macrophages may fuse to form foreign body giant cells, contributing to implant degradation and fibrotic encapsulation.51 Understanding these inflammatory mechanisms is crucial for developing strategies that mitigate cellular damage and promote tissue repair in implant-related muscle injuries.
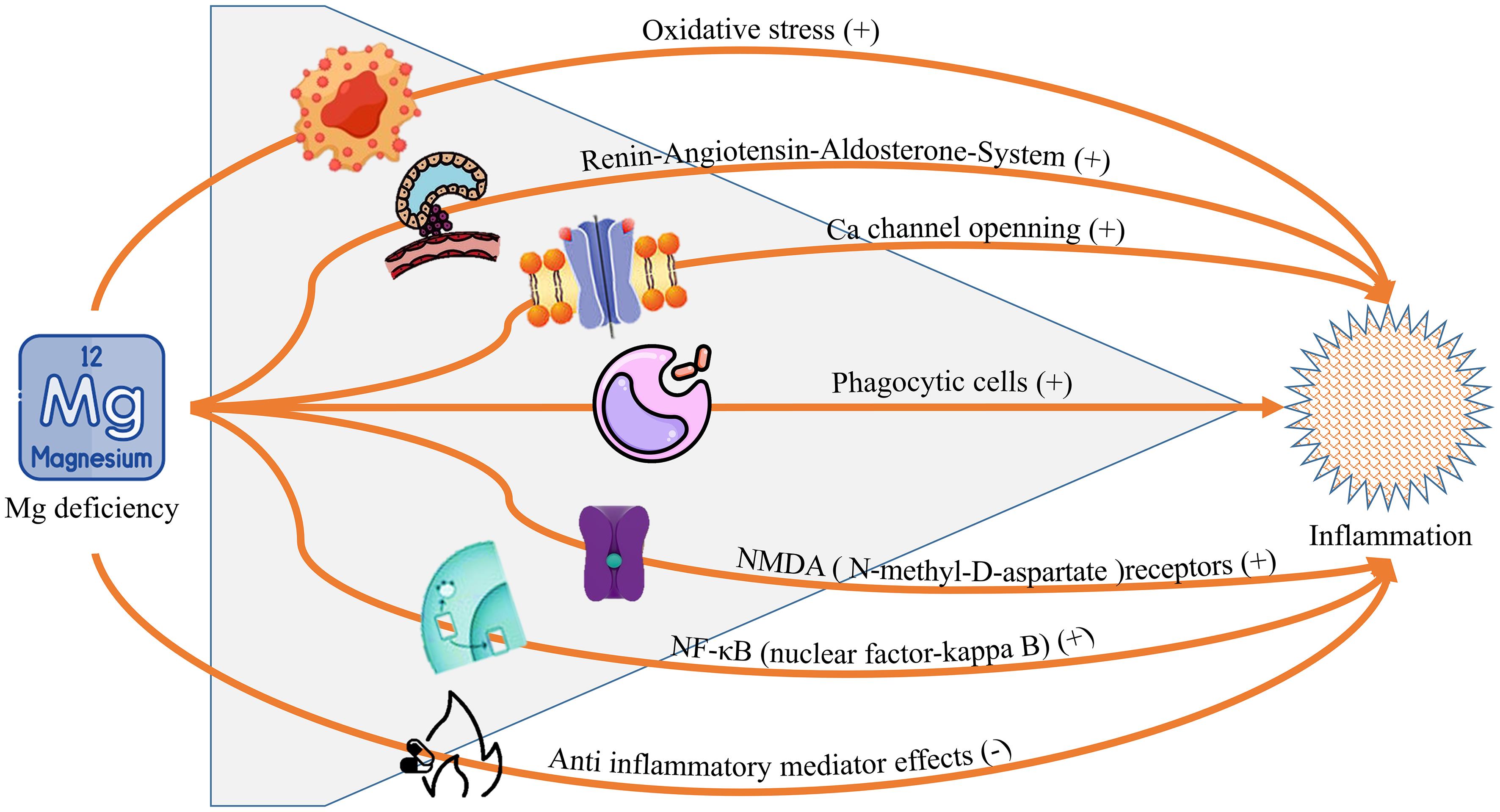
Mg-based implants and their degradation products play a complex role in modulating inflammation. Initially, Mg degradation exacerbates inflammation, but it subsequently promotes immunomodulatory and proangiogenic effects while reducing peri-implant fibrosis.36 Mg ions (Mg2+) can convert macrophages from M0 to M2 phenotype, suppressing pro-inflammatory cytokines and upregulating anti-inflammatory markers.55 This anti-inflammatory effect is dose-dependent and associated with reduced nuclear factor kappa-light-chain-enhancer of activated B cells activation. Silver-containing Mg alloys have shown additional potential as anti-inflammatory implant materials, reducing the need for long-term anti-inflammatory medications.56 However, Mg deficiency can sensitize cells to inflammatory stimuli and contribute to vascular and cellular events during acute inflammation.57 These findings highlight the dual nature of Mg in inflammation, as illustrated in Figure 1 (CC-BY 4.0),58 underscoring its potential as an anti-inflammatory agent in clinical applications.
Fibrosis and muscle atrophy
Muscle degeneration following implantation involves complex processes of fibrosis and atrophy. Fibrosis is characterized by increased collagen deposition and extracellular matrix remodeling, often triggered by transforming growth factor-beta activation.53,59 This process is accompanied by the recruitment of inflammatory cells, particularly neutrophils and macrophages, as well as the production of pro-inflammatory cytokines such as tumor necrosis factor-alpha and C-C motif chemokine ligand 2 / monocyte chemoattractant protein-1.53 Muscle atrophy occurs through the activation of proteasomal and lysosomal pathways, leading to the breakdown of contractile proteins.59 The fibrotic environment poses challenges for drug delivery due to increased tissue stiffness, density, and altered pH.60 While inflammation plays a crucial role in muscle repair, chronic inflammation can result in persistent atrophy and fibrosis, ultimately impairing muscle function.61 Understanding these mechanisms is essential for developing effective therapeutic strategies to combat muscle degeneration.
Mg alloys show promise as biodegradable implants due to their biocompatibility and mechanical properties.62 However, their rapid degradation can limit their clinical application. Controlling the degradation rate is crucial for maintaining implant integrity during tissue healing.63 Although Mg degradation initially exacerbates inflammation, it subsequently promotes immunomodulation and angiogenesis.36 Additionally, Mg implants tend to produce thinner fibrous encapsulation compared to titanium, potentially reducing fibrosis. Various strategies—such as alloying, surface treatments, and structural design—can be employed to control Mg degradation. For instance, strontium phosphate coatings can protect Mg from early degradation while preserving biocompatibility.25 Understanding the relationship between Mg properties and cellular processes is key to designing optimized implants.36 Controlled Mg degradation can provide mechanical support during the early stages of healing while allowing for natural resorption later, eliminating the need for surgical removal.25
Limitations of muscle regeneration
Muscle regeneration is limited by several factors, including mitochondrial dysfunction, cellular senescence, and age-related changes in the immune system. Mitochondrial health is essential for muscle stem cell function and energy production during repair.64 Senescent cells within the muscle niche contribute to a pro-inflammatory environment that impairs regeneration, whereas their removal can accelerate the healing process.65 Aging of the immune system also reduces regenerative capacity, as age-related changes in immune cell populations negatively impact muscle stem cell function and disrupt the inflammatory response to injury.66 Current clinical treatments for severe muscle injuries, such as volumetric muscle loss, are limited, prompting ongoing research into tissue engineering approaches that combine cell therapy, scaffold design, and bioactive factor delivery.67 Addressing these limitations could improve muscle regeneration techniques and enhance outcomes for patients with traumatic or age-related muscle loss.
Mg ions (Mg2+) also play an important role in muscle regeneration and peripheral nerve repair. Mg2+ supplementation enhances mTOR signaling, promoting myogenic differentiation and protein synthesis, both of which are beneficial for counteracting age-related muscle decline.68 Nanosized silk-Mg complexes have shown promise in tissue regeneration, demonstrating angiogenic and anti-inflammatory properties.69 However, non-physiological Mg concentrations may induce oxidative stress in myoblasts, inhibiting membrane fusion and impairing myogenesis.70 In peripheral nerve regeneration, Mg-based biomaterials are considered promising due to their biocompatibility and biodegradability.33,71 While Mg supplementation offers potential benefits for both muscle and nerve regeneration, maintaining appropriate Mg homeostasis is essential for optimal therapeutic outcomes. These findings suggest that Mg could be a valuable component of regenerative medicine strategies for treating muscle dysfunction, though further research is needed to fully elucidate its mechanisms and clinical applications.
Interaction of light alloys with muscle tissue
Biological and mechanical properties of Mg
Mg alloys have attracted significant attention as biodegradable biomaterials for orthopedic applications due to their biocompatibility, favorable mechanical properties, and biodegradability.5,72–74 These alloys offer advantages over traditional metal implants, including the elimination of secondary surgeries and reduced stress-shielding effects.5,74 Mg ions released during degradation promote osteogenesis and angiogenesis, thereby enhancing bone healing.5 However, the rapid corrosion rate of pure Mg in physiological environments presents challenges.74 To address this, various strategies such as alloying, thermal processing, and surface modification have been explored.73,74 These approaches aim to improve mechanical strength, corrosion resistance, and biocompatibility.72,73 Ongoing research seeks to optimize Mg alloys so that their degradation rate matches the timeline of bone tissue healing, positioning them as promising candidates for future orthopedic implants.74
Recent studies have also investigated the interactions of Mg implants with soft tissues and cells. For example, Mg ion implantation on titanium surfaces enhances the adhesion and migration of human gingival fibroblasts via activation of the mitogen-activated protein kinase pathway.75 In bone regeneration, moderate concentrations of Mg2+ (∼4.11 mM) promote osteogenic differentiation and intramembranous ossification.76 While Mg implant degradation in soft tissue initially intensifies inflammation, it later provides immunomodulatory and pro-angiogenic effects, resulting in thinner fibrous encapsulation compared to titanium implants.36 Additionally, surface roughness gradients on Mg implants reveal differential responses from endothelial and smooth muscle cells, where topographical cues can override the biochemical influence of released Mg2+ ions.77 These findings underscore the complex interactions between Mg implants and surrounding tissues, providing insights for optimizing implant design and improving biocompatibility in diverse medical applications.
Corrosion of Mg and muscle degeneration
Mg alloys are promising materials for biomedical applications, particularly as orthopedic implants, due to their biocompatibility and mechanical performance.78 However, their high degradation rate in physiological environments remains a major challenge.78,79 The corrosion of Mg alloys typically occurs in multiple stages, including rapid initial corrosion, a steady state phase, and accelerated corrosion.79 This progressive degradation can result in the loss of mechanical strength and ductility over time.80 To mitigate these issues, researchers are developing strategies such as alloying and surface modification.78 Bioinspired surface designs have emerged as a promising means of controlling corrosion behavior while enhancing implant functionality.81 Computational modeling and experimental studies are also being used to predict and understand corrosion behavior in physiological conditions.80
Surface modifications of Mg-based implants are essential for addressing corrosion-related complications and improving biocompatibility. A variety of approaches—including bulk and surface modifications—have been explored to reduce corrosion rates and enhance mechanical integrity.82 These efforts aim to limit excessive inflammatory responses and prevent implant-associated infections, both of which are common complications in orthopedic procedures.83 Functional coatings, including metal oxides, polymers, and composite materials, have shown potential in regulating degradation and improving biological performance in cardiovascular stents.84 However, the complex interplay between Mg corrosion and biological responses—such as the generation of reactive intermediates and redox interactions with cells and biomolecules—requires a comprehensive understanding of metal–cell interactions.85 Advances in surface modification may help reduce inflammation and fibrosis in muscle tissue, although further studies are needed to fully elucidate their effects.
Comparison of Mg alloys with other materials
Mg alloys have gained widespread interest in various fields due to their unique material properties. These lightweight metals offer high strength, effective shock absorption, and good corrosion resistance.86 In biomedical contexts, Mg alloys are biodegradable and support bone tissue regeneration, with a Young’s modulus that more closely matches natural bone compared to other metallic implants.74 However, their rapid corrosion in physiological environments presents significant challenges, necessitating improvements through alloying, machining, and coating techniques.74,87 Beyond biomedicine, Mg alloys also show promise in electromagnetic shielding applications. Their shielding performance is influenced by grain size, texture, alloying elements, and secondary phases.88 Recent developments have led to the creation of high-performance Mg alloys for shielding applications, such as Mg–Zn–Y–Ce–Zr and Mg–Sn–Zn–Ca–Ce systems.88 These advancements highlight the versatility and potential of Mg alloys in various engineering applications.
As biomedical materials, Mg alloys are particularly promising due to their biocompatibility, biodegradability, and mechanical properties that resemble those of bone.62,89 Their biodegradability eliminates the need for implant removal surgery, and the released Mg ions stimulate bone formation while providing antimicrobial effects.45,74 Nonetheless, their rapid corrosion in physiological environments poses obstacles to maintaining structural integrity during tissue repair.62,74 To overcome this, researchers are developing methods to control the degradation rate, including alloy design, microstructural modifications, surface treatments, and the use of Mg-based bulk metallic glasses.45,62,89 These approaches aim to balance degradation and bone healing rates, while addressing limitations associated with conventional metallic implants such as stress shielding and toxic ion release.45,74
Clinical and experimental findings
Clinical findings
Clinical studies on Mg-based implants for orthopedic applications have shown promising results, with excellent patient outcomes and no need for implant removal.90,91 These implants offer advantages such as biodegradability, reduced stress shielding, and enhanced bone strengthening.92 Mg implants promote bone formation, angiogenesis, and exert immunomodulatory effects within the bone microenvironment.91 However, challenges remain, including high corrosion rates, unpredictable degradation, and potential structural failure.92In vivo studies have generally found that Mg implants cause only mild to moderate inflammatory reactions, though the timeline of foreign body giant cell formation varies across studies. Further research is needed to better understand immunological responses to Mg implants and how implant characteristics, such as size, shape, and alloy composition, affect degradation kinetics and host responses.93
Skeletal muscle regeneration is a complex process involving multiple cell types and signaling pathways.94 Muscle satellite cells play a crucial role in muscle repair and regeneration.95 However, aging and pathological conditions can impair the function of muscle satellite cells, reducing regenerative capacity.95,96 In cases of volumetric muscle loss, persistent infiltration of neutrophils and natural killer cells can hinder muscle stem cell-mediated repair. Additionally, cellular senescence—a state of irreversible cell cycle arrest—accumulates in aging tissues and contributes to muscle degeneration.97 To address these challenges, various strategies for skeletal muscle tissue engineering have been developed, including the use of stem cells, biomaterials, and biomolecules.94 Understanding the mechanisms of muscle regeneration and the aging of stem cells is essential for developing effective therapies.
Recent clinical studies further highlight the potential of Mg-based implants in orthopedic applications due to their biodegradability and capacity to enhance tissue regeneration. Trials have reported encouraging results for Mg implants in bone fracture fixation, eliminating the need for removal surgery.90 These implants promote bone formation, angiogenesis, and exert immunomodulatory effects within the bone microenvironment.91 However, variability in degradation rates and potential hydrogen gas formation remain concerns.92 Strategies such as alloying and surface coating have been explored to regulate degradation and improve biocompatibility.25,98,99 While Mg implants offer advantages like reduced stress shielding and improved bone integration, issues such as unexpected degradation and mechanical failure still pose challenges. Ongoing research focuses on optimizing Mg compositions and surface modifications to overcome these limitations and broaden clinical applications.91,92 A summary of this section is provided in Table 2.9,12–14,22,100
Biomaterial properties with clinical findings
| Implant type | Clinical findings | Long-term impacts |
|---|---|---|
| Ti alloys9,12 | High biocompatibility, stability | Chronic inflammation, risk of fibrosis |
| Co-Cr alloys13,14 | Abrasion resistant, long life | Tissue hardening, biocompatibility problems |
| Mg alloys22,100 | Biodegradable, reduces inflammation | Controlled degradation is difficult, and mechanical strength is low |
Experimental studies
Mg-based implants demonstrate significant potential for biomedical applications, particularly in fracture treatment, due to their biodegradability and bone-healing capabilities.101 However, their high corrosion rate presents challenges such as unpredictable degradation and potential cytotoxicity.92,102–104 Animal models—including rats, rabbits, and pigs—have been widely used to evaluate the in vivo performance of Mg-based materials.92,101 At the cellular level, Mg implants influence gene and protein expression, cell adhesion, and immune responses.38 Mg deficiency can lead to inflammation, characterized by elevated cytokine production and oxidative stress.105 While in vitro studies have extensively assessed the biocompatibility of Mg-based materials, further in vivo research is essential to fully understand their biological effects and optimize their clinical applications.92,101
Recent studies have investigated various surface modification techniques aimed at improving the performance of Mg implants in biomedical settings. Sandblasting has been shown to significantly alter surface roughness, hardness, and corrosion behavior, with pressure being a critical variable in these outcomes.106 Polymer coatings applied to plasma electrolytic oxidation surfaces have been investigated to enhance both biocompatibility and corrosion resistance.107 Laser surface modification techniques—including laser melting, alloying, cladding, texturing, and shock peening—have also demonstrated potential in improving surface characteristics such as corrosion resistance and cellular response.108 These modifications aim to address the primary limitation of Mg implants: their rapid corrosion in physiological environments. Both bulk and surface modifications have been studied to improve corrosion and corrosion-fatigue resistance, ultimately preserving mechanical integrity during tissue healing.82
Magnesium-based implants continue to show promise in various biomedical applications due to their biodegradability and ability to support bone regeneration and vascular remodeling.92,109 Animal studies involving rats, rabbits, dogs, and pigs have been conducted to assess the biocompatibility, degradation behavior, and osteogenic potential of these materials.99,101,109 Results indicate good in vivo biocompatibility and osteogenic activity, with no adverse tissue reactions observed near the implants.109 Nonetheless, challenges such as rapid degradation in biological fluids remain, which can compromise mechanical integrity before complete healing is achieved.110 To mitigate these issues, researchers have pursued approaches including alloying, surface modification, and coating techniques to enhance corrosion resistance and better control degradation rates.109,110 Despite the promising data, standardization of animal models and study designs is necessary to facilitate clinical translation.101
Discussion and future perspectives
Role of Mg implants in muscle degeneration
Mg-based implants have attracted significant attention in orthopedics due to their biocompatibility, biodegradability, and mechanical properties that closely resemble those of natural bone.72,100 These implants offer several advantages over permanent metallic implants, such as eliminating the need for secondary surgeries.72 However, the rapid corrosion of Mg alloys in physiological environments presents a challenge, potentially leading to early fractures and surgical failures.111 To address this issue, various strategies have been explored, including composite preparation, surface modification, and polymer coatings.72,111 These approaches aim to retard degradation and enhance bioactivity. Despite considerable progress in alloy development and fabrication techniques, a significant limitation remains: the mismatch between bone healing rates and alloy degradation. This issue hinders the widespread clinical application of Mg-based implants.112 Ongoing research focuses on optimizing degradation behavior, enhancing mechanical properties, and improving biocompatibility to facilitate the clinical transition of Mg-based implants in orthopedics.100,112
Mg alloys are emerging as promising biodegradable biomaterials for bone implants, offering an alternative to traditional materials such as stainless steel, cobalt-chromium, and titanium alloys.113,114 These conventional materials often lead to stress shielding and ion release and require secondary surgeries for removal.45 Mg alloys, by contrast, are biocompatible, possess mechanical properties similar to bone, and biodegrade over time, eliminating the need for implant removal.45,115 However, the rapid corrosion of Mg alloys in body fluids presents a significant challenge, potentially compromising the integrity of the implants.114,115 Researchers are exploring strategies to control Mg degradation, such as incorporating alloying elements, surface treatments, and developing Mg-based bulk metallic glasses.45,113 These efforts aim to optimize corrosion resistance while maintaining the beneficial properties of Mg alloys for bone tissue regeneration. The properties of other biomaterials in comparison to Mg alloys are summarized in Table 3. Although Mg alloys hold great promise due to their bioresorbability, further optimization is required to improve their mechanical properties and control their degradation rate.
General bio-comparison of Mg alloys with other biomaterials
| Property | Ti alloys | Co-Cr alloys | Stainless steel | Mg alloys |
|---|---|---|---|---|
| Biodegradation | None | None | None | High |
| Inflammation risk | Middle | High | High | Low |
| Mechanical stability | High | High | High | Middle |
| Fibrosis development | Diffuse | Diffuse | Diffuse | Less |
| Immunomodulation | None | None | None | High |
Gaps in the literature
Mg implants have shown considerable potential in orthopedic applications due to their degradability and their ability to promote bone regeneration. These implants have been shown to enhance angiogenesis, modulate immune responses, and reduce peri-implant fibrosis in soft tissue.36 Mg ions play a crucial role in regulating bone metabolism by promoting osteogenesis and inhibiting osteoclast activity.116 In fracture treatment, Mg-based implants have demonstrated the ability to promote healing, though standardized preclinical models are still lacking.101 Notably, biodegradable Mg implants have shown potential in alleviating medication-related osteonecrosis of the jaw by enhancing angiogenesis through the upregulation of vascular endothelial growth factor and calcitonin gene-related peptide mediated pathways.117 While these findings highlight the promising applications of Mg implants, further research is necessary to fully understand their long-term effects and optimize their clinical use.
Mg alloys also exhibit promising immunomodulatory effects in tissue regeneration. Initially, the degradation of Mg implants may exacerbate inflammation,36 but over time, they promote a shift from pro-inflammatory M1 macrophages to anti-inflammatory M2 macrophages.118,119 This transition is crucial for reducing chronic inflammation and enhancing tissue healing. Mg ions have been found to decrease the expression of pro-inflammatory markers, even in mildly inflammatory environments.119In vivo studies indicate that Mg alloys can stimulate macrophage polarization, increase vascularization, and reduce fibrous tissue formation when compared to titanium implants.36,120 However, the osteogenic effects of Mg alloys remain unclear, with some studies showing enhanced osteoblast maturation while others report decreased osteoblast activity.118,120 These conflicting findings highlight the potential of Mg alloys to create a favorable environment for tissue regeneration through immunomodulation.
The biomechanical interactions between implants and surrounding tissues are crucial for implant stability and success.121 Understanding tissue biomechanics is essential for developing realistic models and improving implant performance, as highlighted in studies on penile prostheses.122 Advanced bioprinting techniques, such as assembled cell-decorated collagen, are being explored to create implants with properties similar to native musculoskeletal tissue, promoting functional recovery in cases of volumetric muscle loss.123 The graft-tissue interface plays a critical role in implant success, and a better understanding of these interactions could lead to improved graft designs with enhanced biocompatibility.35 However, further investigation is needed into the biomechanical properties of specific tissues, such as the corpora cavernosa and corpus spongiosum, to develop more accurate computational models and preclinical testbeds for implant testing.122
Surface modifications for Mg implants
Recent research has focused on controlling the corrosion rate of Mg alloys through surface modifications and coatings. Various approaches have been explored, including conversion and deposition coatings, mechanical treatments, and novel alloy designs.25,124 Surface coatings, particularly those using natural biopolymers, have shown promise in improving corrosion resistance, cell adhesion, and biodegradability.125 Protective and functional osseoconductive coatings have been developed to enhance biostability and stimulate tissue ingrowth.126 Researchers have also investigated the effects of porous structures, phase structures, and grain sizes on the degradation behavior of Mg alloys.25 Despite progress, challenges remain in achieving optimal corrosion control for Mg alloys. Future studies should focus on hybrid treatments that combine innovative biomimetic coatings with mechanical processing, along with rigorous testing and characterization to assess their efficacy.124
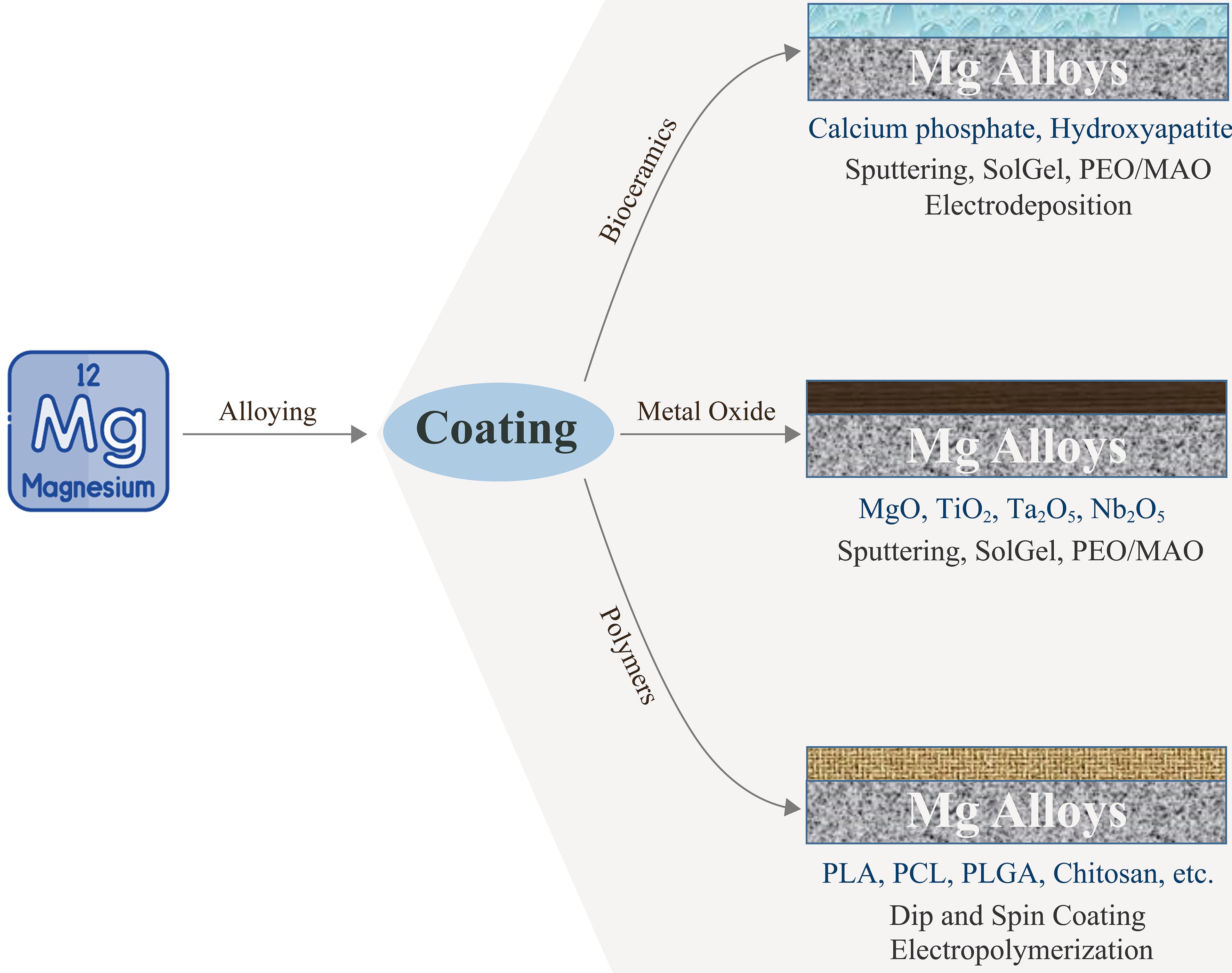
Mg alloys hold promise as biodegradable implants but face challenges due to their rapid corrosion in physiological environments.40,127 To address this, various surface modification techniques have been investigated. Plasma electrolytic oxidation combined with hydroxyapatite particles creates a ceramic-like coating that enhances corrosion resistance and bioactivity.128 Polymer coatings, including sol-gel, synthetic aliphatic polyesters, and natural polymers, have also been explored to improve corrosion resistance and biocompatibility.40 Bioabsorbable polymers are particularly promising due to their biocompatibility and potential for drug encapsulation.129 Multilayer hybrid coatings that combine chemical pretreatment, inorganic hydroxyapatite coating, and protein-based polymer coatings may offer a promising approach to optimize corrosion resistance and biocompatibility.127 These surface modification strategies aim to control degradation rates while preserving the beneficial properties of Mg alloys for orthopedic applications. A comparison of the effects of these modification types can be found in Table 4 and Figure 2 (CC-BY 4.0).130–136 Future studies should focus on developing hybrid coatings that combine mechanical reinforcement with bioactive properties, ensuring both stability and controlled bio-resorption.
Comparison of the effects of different modification types
| Coating method | Advantages | Disadvantages |
|---|---|---|
| Plasma electrolytic oxidation131,132 | High corrosion resistance, biocompatible | Coating homogeneity difficult |
| Hydroxyapatite coating133,134 | Increases bone bonding | High cost |
| Biopolymer coatings135,136 | Provides controlled degradation, adds flexibility | Long-term stability unknown |
MAO, micro-arc oxidation; Mg, magnesium; PCL, polycaprolactone; PEO, plasma electrolytic oxidation; PLA, polylactic acid; PLGA, poly(lactic-co-glycolic acid.
Conclusions
The choice of implant materials significantly affects post-implant muscle degeneration, influencing inflammation, fibrosis, and tissue regeneration. Traditional materials such as titanium, cobalt-chromium, and stainless steel offer mechanical stability but often induce chronic inflammation and fibrosis. In contrast, Mg alloys demonstrate significant potential in promoting muscle healing due to their bioresorbability and immunomodulatory properties. Degradation products of Mg reduce inflammation, enhance angiogenesis, and support tissue regeneration—benefits that are largely absent in conventional metallic implants.
Nevertheless, the uncontrolled degradation of Mg remains a major challenge, as it can compromise the mechanical integrity of the implant. Advances in alloy composition and surface modification techniques are crucial for optimizing the performance of Mg implants. Future research should focus on balancing Mg’s bioactivity and its controlled resorption rates, ensuring it becomes a reliable alternative to permanent metallic implants. If these challenges are successfully addressed, Mg alloys could offer a next-generation solution that bridges the gap between mechanical stability and biological functionality in orthopedic applications.
Declarations
Acknowledgement
None.
Funding
No funding was received for this work.
Conflict of interest
The authors declare no conflict of interest regarding the publication of this manuscript.
Authors’ contributions
Study concept and design (BC), experiment performance, and data collection (YS). Both authors read and approved the final manuscript.

 Author information
Author information