Introduction
Myopia, commonly referred to as nearsightedness, represents a significant refractive error of the eye, in which distant objects appear blurred while near objects remain clear.1 This condition primarily develops during childhood and early adulthood and results from excessive elongation of the eyeball, causing images to focus in front of the retina rather than directly on it.2 Although this optical aberration may seem straightforward, myopia is a complex trait influenced by genetic predisposition, environmental factors, and individual behaviors, all of which contribute to its onset and progression.3
In recent years, myopia has emerged as a global public health concern, with its prevalence rising alarmingly across different regions, particularly in East and Southeast Asia, where studies report rates as high as 80–90% among young adults.4 Such statistics indicate that by 2050, nearly half of the world’s population could be affected by myopia.5 This soaring prevalence poses substantial public health challenges due to the increased risk of severe ocular conditions, such as myopic macular degeneration, retinal detachment, cataracts, and glaucoma—leading causes of vision impairment and potential blindness.6,7
The rapid increase in myopia prevalence has prompted further investigation into its underlying causes and potential interventions. Compelling evidence from numerous studies underscores the role of both genetic and environmental factors in the development and progression of myopia.8 While genetic factors confer susceptibility, contemporary lifestyle elements, including extensive educational demands and other near-work activities, are also suspected to be critical contributors.9
Myopia is typically defined by a spherical equivalent (SE) refractive error of ≤−0.5 diopters (D), with any degree of myopia increasing the risk of adverse ocular tissue changes.10 The risk rises substantially at higher levels of myopia (high myopia, SE worse than −5.0 D or −6.0 D) and in pathological myopia, which involves secondary retinal changes that can lead to irreversible visual impairment or blindness.11,12 Consequently, the widespread need for optical correction, coupled with the ocular health risks associated with myopia, underscores the urgency of implementing both primary and secondary preventive measures.2,13–15 These measures aim to delay the onset of myopia in children and slow its progression in later childhood.
Modern myopia control strategies span a range of approaches, including progressive addition lenses, topical atropine, orthokeratology lenses, and multifocal contact lenses, each offering potential benefits for preserving ocular health.16–20 Staying informed on the latest findings regarding myopia’s etiology and treatment strategies enables stakeholders to actively contribute to safeguarding visual health and improving the quality of life for those affected by this pervasive condition.
Myopia can be classified into two types based on its underlying mechanism: axial myopia and refractive myopia.21 The more common form, axial myopia, results from elongation of the eye’s axial length, preventing incoming light from focusing directly on the retina. In contrast, refractive myopia is characterized by an increased refractive power of the eye’s optical components without significant axial elongation.22 This form of myopia is primarily attributed to alterations in the refractive index or curvature of the cornea or lens. Individuals with refractive myopia experience a focusing issue in which light converges at a point anterior to the retina, resulting in blurred vision for distant objects. Such refractive anomalies may arise due to developmental factors, genetic predisposition, or secondary conditions affecting the lens, such as nuclear sclerosis or other lenticular changes.23 This review primarily focuses on the more common axial myopia.
This review aimed to provide an in-depth examination of the latest developments in the epidemiology, pathogenesis, diagnosis, and treatment of myopia. It is intended to offer general practitioners, pediatricians, and ophthalmologists a comprehensive and up-to-date framework for diagnosing and managing myopia.
Epidemiology of myopia
Myopia has emerged as the most prevalent ocular disorder worldwide, posing significant public health challenges. Epidemiological data illustrate that myopia affects approximately 28.3% of the global population, translating to nearly two billion individuals.24 Alarmingly, projections suggest a surge to nearly 4.8 billion individuals, or 49.8% of the global population, by 2050.5 This rising trend is accompanied by an increase in high myopia cases, from 4.0% to 9.8% of the global population within the same timeframe.25
Global prevalence trends
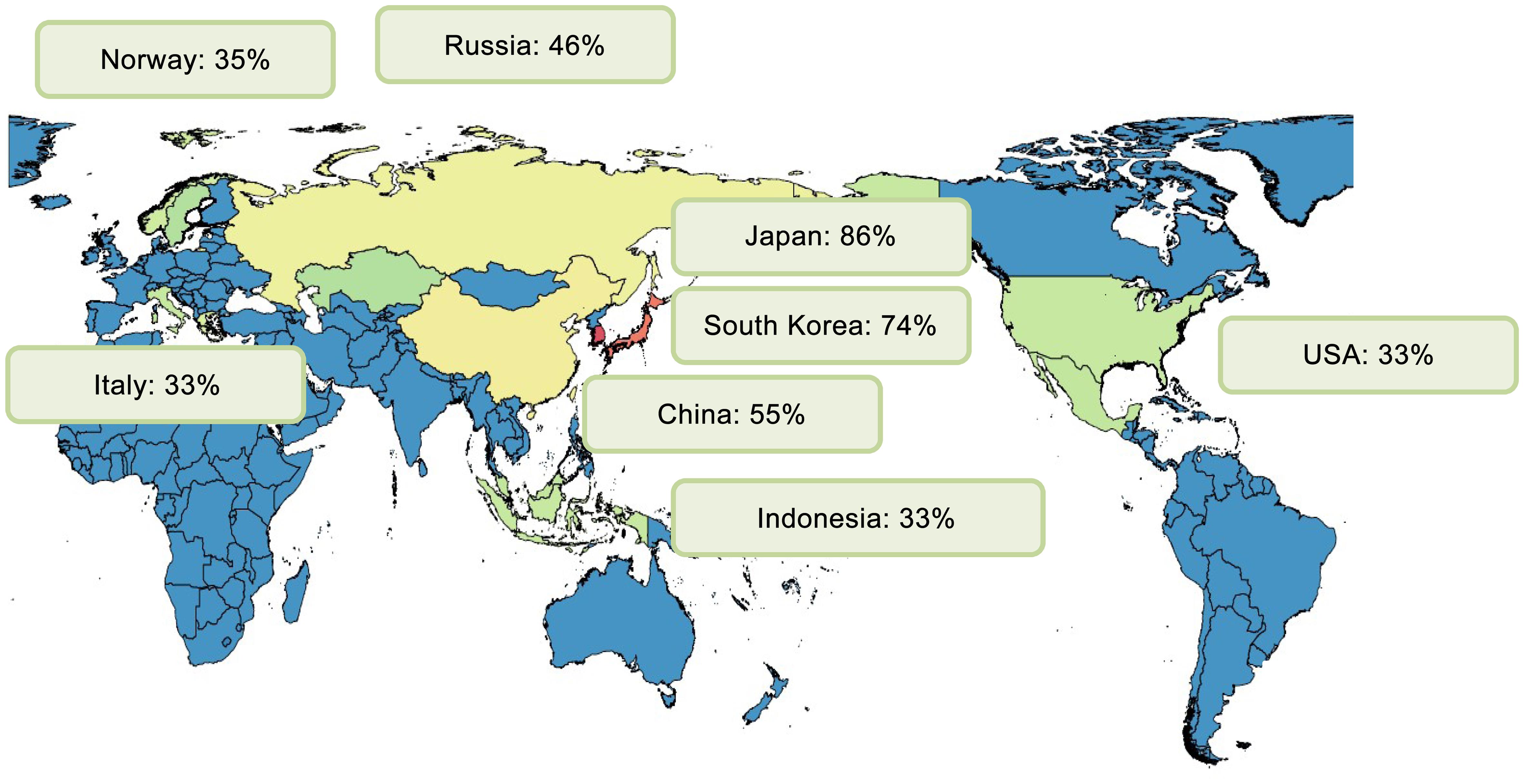
As shown in Figure 1, the prevalence of myopia exhibits considerable geographical variability. In urbanized regions of East and Southeast Asia, such as Singapore, Japan, South Korea, and China, myopia prevalence is notably high. Conversely, regions such as Central Africa and rural areas in Nepal, South America, and parts of India maintain substantially lower prevalence rates. Western countries, including the United States and several European nations, have also experienced a notable rise in myopia prevalence over recent decades, likely influenced by lifestyle changes and urbanization.26,27
Asia
China
In China, the prevalence of myopia among adolescents has been widely studied across various regions, with significant findings elucidating contributing factors. In Chongqing, a cross-sectional study of schoolchildren revealed an alarmingly high myopia prevalence, recorded at 73.1% among elementary school students and 81.8% among junior middle school graduates. The study implicated academic pressures, insufficient outdoor activity, and poor eye care habits as significant contributors to the myopia epidemic.28 Similarly, in Hangzhou, the overall prevalence of myopia among children and adolescents was reported as 55.3%, with rates escalating to 85.0% among senior high school students.29 Factors such as prolonged exposure to electronic screens, inappropriate lighting, and incorrect reading postures were identified as influential in the development of myopia.
In Nantong, a cross-sectional survey of adolescents aged 12–19 found a myopia prevalence intrinsically linked to uncorrected refractive errors. The study reported a total uncorrected refractive error prevalence of 23.7%, with myopia being a major risk factor.30 Notably, lifestyle habits such as increased daily use of electronic devices exacerbated these conditions. In the Ningxia Hui Autonomous Region, the prevalence of myopia among students was documented at 27.3%, with lifestyle behaviors and physical activity strongly associated with vision health.31 A unique study assessed the relationship between outdoor artificial light at night and myopia, identifying a positive, nonlinear association, suggesting that controlling light pollution could help mitigate myopia incidence in adolescents.32 In Guangzhou, a metropolitan area in southern China, myopia affects 73.1% of children aged 15, indicating a significant public health concern.33 In contrast, Hong Kong presents a myopia prevalence of 25% among children aged six to eight, with higher rates observed among boys.34 This trend aligns with reports from Taiwan, where myopia prevalence among schoolchildren increased dramatically from 5.8% in 1983 to 21% by 2000. In Japan, schoolchildren exhibit some of the highest rates globally, with prevalence recorded at 76.5% among elementary students and 94.9% among junior high students.35 This geographic variability within a single country underscores the influence of urbanization and lifestyle factors.
In Hong Kong, the surge in myopia among children aged six to eight emphasizes both genetic predispositions and environmental factors, such as intensive educational demands.34
Taiwan reveals similar insights; risk factors for myopia in Taipei’s schoolchildren include parental myopia, frequent near-work activities, and limited outdoor time.36
Furthermore, a meta-analysis investigating the association between dry eye disease and myopia highlighted a 45.1% prevalence of dry eye disease symptoms among myopic individuals, underscoring the importance of addressing ocular surface health in myopia management strategies.37
Singapore
In Singapore, the prevalence of myopia in children is remarkably high, with contributing factors including age, sex (male), reading habits, height, and parental myopia. A cross-sectional study of Singaporean Chinese children aged seven to nine found that those who read more than two books per week had longer axial lengths, indicative of greater myopic progression.38,39 Conventional risk factors such as increased vitreous cavity depth are well documented, though certain anterior segment parameters appear to follow different growth mechanisms.
Japan and South Korea
High myopia, a growing concern due to its pathological potential, is prevalent in Japan and South Korea, with strong associations with higher education levels and urban living.35,40–42 These findings reveal consistent age-associated increases, with hyperopia diminishing as myopia escalates during adolescence.
Europe
United Kingdom
The European eye epidemiology consortium reports a rising trend in myopia across Europe, with prevalence increasing from 17.8% to 23.5%.43–45 This growth is partially attributed to educational advancements, though it goes beyond just academic exposure to suggest environmental interplay.
Norway and Denmark
In Norway, the prevalence of myopia among young and middle-aged adults is 35%, with women aged 20–25 showing higher rates than men.46,47 Denmark, historically exhibiting stable prevalence, has reported a decline in low myopia, attributing changes to variables such as educational attainment and cognitive performance.48,49
America
United States
In the United States, myopia prevalence varies across ethnic: 35.2% among non-Hispanic whites, 28.6% among non-Hispanic blacks, and 25.1% among Mexican Americans.50,51 The National Health and Nutrition Examination Survey reports a 33.1% myopia rate among adults, emphasizing the need for clinical interventions and eyewear solutions.52
Brazil
In Brazil, myopia prevalence follows a unique age-related pattern, with the highest rates observed among individuals aged 30–39.53 Myopia predominantly affects urban populations, where lifestyle factors and occupational demands likely contribute to its development.54
The epidemiology of myopia across these regions reflects complex interactions between genetic, environmental, and lifestyle factors. Different countries and regions experience varied prevalence and risk profiles, underscoring myopia’s multifaceted nature. Each regional demographic necessitates a tailored strategy for myopia management, highlighting the importance of early intervention and public health education to curb this visual epidemic.
Risk factors
Several genetic, environmental, and lifestyle factors contribute to the development and progression of myopia.
Initiating factor for myopia
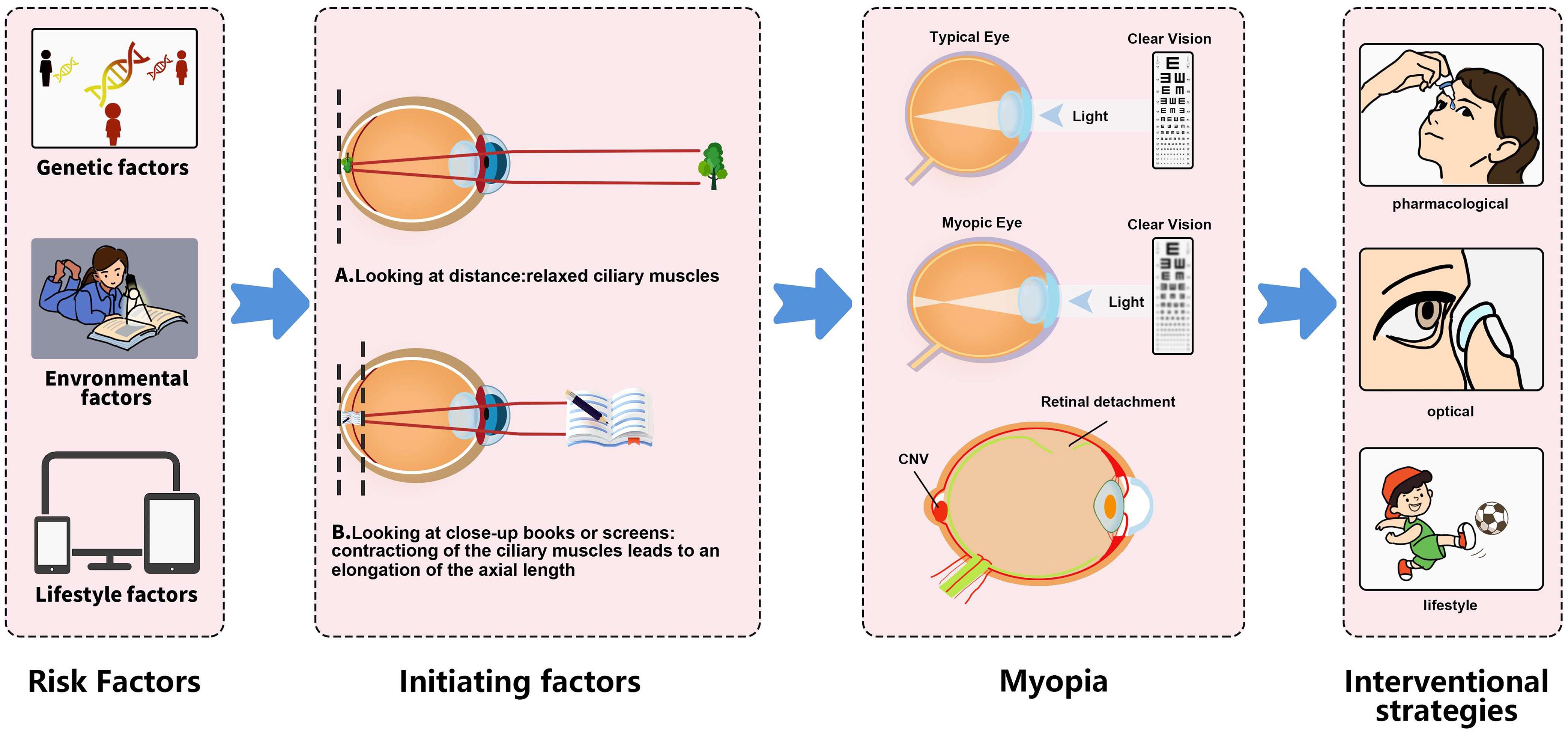
As shown in Figure 2, contraction of the ciliary muscle, leading to an elongation of the axial length, may serve as an initiating factor in the onset of myopia.55 By comparing the axial lengths of the eye under normal ciliary muscle conditions to those when the muscle is in a relaxed state, a slight shortening of the eye’s axial length was observed upon muscle relaxation, while a slight elongation was noted when the muscle was tense. Furthermore, an investigation into the force distribution of ciliary muscle contraction on the eyeball was conducted using computerized mechanical modeling.55
When viewing distant objects, the ciliary muscles in the eye are in a relaxed state (A). However, when looking at nearby objects, such as reading a book or viewing a smartphone screen, the ciliary muscles contract, causing the axial length of the eye to increase (B). This change is an initiative factor in the development of myopia.
Genetic associations
Numerous studies highlight the genetic basis of myopia, identifying specific genes associated with its development. The HTRA1 gene, known for its association with age-related macular degeneration, has shown significant associations with myopia in different subpopulations. Specifically, SNP rs11200647 is significantly linked to myopia, suggesting shared genetic components between myopia and other ocular conditions.56 Similarly, a genome-wide association study identified the LILRB2 gene as a new risk locus associated with pathological myopia, elucidating its role in lipid metabolism disorders, which impair choroidal function and promote myopic degeneration.57 Twin studies and familial aggregations further corroborate this genetic link. Recent advances in genome-wide association studies have identified multiple loci associated with refractive errors, highlighting the polygenic and heterogeneous nature of myopia. These include crucial genes such as PAX6, involved in eye development, and GJD2, associated with retinal neuronal communication.58–61 Rare genetic variants have been implicated in high myopia, indicating a potential path for targeted therapeutic interventions. Notably, genes like ARR3, BSG, and LEPREL1 have been linked to high myopia, with their inheritance patterns predominantly following an autosomal dominant model, except for a few with autosomal recessive and X-linked patterns.62–64
Gene-environment interactions
Recent investigations have elucidated gene-environment interactions in myopia, offering insight into the condition’s complex etiology.65 The etiology of myopia is a multifactorial interplay involving genetic predispositions, environmental exposures, and resultant anatomical changes within the eye. A comprehensive understanding of these factors is crucial for developing preventive and therapeutic strategies to mitigate the burden of myopia in diverse populations.
Gene-environment interactions play a critical role in myopia development. For instance, interactions between genetic variants and educational attainment were observed, indicating that higher education levels may potentiate the effect of myopia risk alleles.66 Additionally, genes such as APLP2 in humans and animals show variations in refractive error development, influenced by time spent reading and engaging in near work.67 Other studies highlight the pervasive nature of gene-gene and gene-environment interactions, revealing a complex interplay between genetic predispositions and environmental exposures.68
Ethnicity and geographic variations
The prevalence of myopia varies significantly across different ethnicities and geographical regions. For example, higher myopia prevalence is observed in urban populations compared to rural counterparts, with factors like parental education level influencing prevalence rates.69 Older adults in various minority ethnic groups in China show differing myopia prevalence, which is linked to their ethnic backgrounds, suggesting environmental influences like altitude and lifestyle.70
Thin central corneal thickness
Children with reduced central corneal thickness (CCT) are observed to experience more rapid progression of myopia and axial length (AL) elongation.71 This trend is consistently noted across both Chinese and Caucasian pediatric cohorts, supporting its pathophysiological plausibility. Previous studies have described a negative correlation between corneal hysteresis and axial elongation in children, which is statistically associated with CCT, further implying an inverse relationship between CCT and myopia progression in young individuals.72 An additional hypothesis suggests a positive association between CCT and scleral thickness, considering the sclera as a critical pathway through which visual signals impact myopia progression. Scleral remodeling, a key factor in myopia development, results in a thinner sclera, thereby facilitating axial elongation. Therefore, reduced CCT suggests decreased scleral thickness and stiffness, increasing the risk of myopia progression.73 Consequently, CCT could potentially act as a surrogate marker for scleral thickness and a predictor of the velocity of myopia progression. The association between reduced CCT and accelerated myopia progression highlights the importance of early myopia control interventions. It is advisable for medical practitioners to consider shorter follow-up intervals for children with thin CCT, along with recommendations for increased outdoor activities and reduced screen time.71,74
Dietary factors
The relationship between dietary macronutrient intake and myopia remains inconclusive, showing no significant associations with carbohydrates, proteins, or fats.75 However, fresh fruit intake appears protective against myopia development, particularly in males, while excessive consumption of sugary beverages increases myopia risk, especially in females.75
Educational and workload factors
Both genetic predispositions and environmental factors contribute significantly to the etiology and progression of myopia. The environmental landscape plays a critical role in myopia development, with extensive near work and educational demands acting as key factors. Academic pressures and lifestyle choices significantly affect myopia progression. Among schoolchildren in Chongqing, China, time spent on homework, attending out-of-school courses, and insufficient outdoor activities were positively correlated with myopia prevalence, suggesting that academic workloads and reduced time spent outdoors are key lifestyle risk factors.28 Similarly, in Hangzhou, extended periods spent on near work and inappropriate lighting conditions were significant contributors to myopia progression.29
Outdoor activities and screen time
Outdoor activities have a protective effect against myopia. Exposure to bright outdoor light is believed to stimulate the release of retinal dopamine, which helps reduce excessive eye growth. Interventions to increase outdoor activity have demonstrated efficacy in reducing myopia progression in children, as evidenced by randomized controlled trials in Taiwan and China.
Time spent outdoors has been consistently reported as a protective factor against myopia. Lack of sufficient outdoor activity is a behavioral risk factor contributing to myopia and its related complications, such as myopic maculopathy and retinal detachment.76 Children’s lifestyle habits, including excessive time spent on electronic devices and inadequate eye care, contribute to the high prevalence of myopia and other refractive errors in urban areas.77
The multifactorial nature of myopia necessitates a comprehensive understanding of its genetic, environmental, and lifestyle determinants to formulate effective prevention and intervention strategies.
Age-specific trends and early-onset myopia
Age-specific analyses reveal an alarming shift towards earlier onset of myopia, with significant public health implications. Early-onset myopia, defined as developing before school age, is increasingly prevalent, with children exhibiting rapid progression to high myopia in adulthood. This trend is concerning because high myopia heightens the risk of complications such as myopic maculopathy, retinal detachment, and glaucoma. The prevalence of early-onset myopia underscores the critical need for early intervention strategies and preventive measures during childhood.
The burgeoning issue of early-onset high myopia (eoHM) presents a noteworthy demographic shift with significant implications for ocular health.78 This review synthesizes the genetic underpinnings, environmental influences, and clinical characteristics of eoHM, highlighting advances in genetic research and potential biomarkers for early detection and management.
The quest to unravel the genetic architecture of high myopia, particularly in young cohorts, has yielded substantial advancements. Šenk et al.79 delineated the genetic basis of high myopia in Slovenian children, identifying genetic causes in 61.1% of cases, with conditions such as Stickler’s syndrome and retinal dystrophies linked to genes like CACNA1F and RPGR. Similarly, Sánchez-Cazorla et al.80 underscore the polygenic nature of eoHM, with pathogenic and likely pathogenic variants comprising 9% of identified mutations, furthering our understanding of this complex phenotype. These findings resonate with studies from Shaanxi province, China, where Lu Ye et al.81 pinpointed mutations in genes such as ARR3 and P3H2, contributing to the intricate genetic landscape of eoHM.
Understanding eoHM requires a nuanced view of genetic predisposition and environmental interplay. Chamarty et al.82 conducted a retrospective analysis highlighting that, despite a high prevalence (63%) of parental myopia among affected individuals, no additional risk was attributable solely to parental refractive status. This suggests that while genetic predisposition plays a significant role, external variables may not significantly exacerbate early myopia in preschool years. The multifaceted nature of eoHM progression is further illustrated by Matsumura et al.,78 who identified prematurity and laser treatment for retinopathy of prematurity (ROP) as critical risk factors influencing myopic outcomes.
Distinctive biometric trends elucidate the clinical spectrum of eoHM. Yum et al.83 reported that biometric markers such as SE and AL are pivotal in determining progression rates in preschoolers, with greater baseline myopic SE and longer AL serving as predictors of progression. This aligns with observations by Jiang et al.,84 who documented varied patterns of choroidal thickness among eoHM subtypes—indicative of differing pathological mechanisms like familial exudative vitreoretinopathy and Stickler syndrome—alluding to potential biomarkers for noninvasive diagnosis and personalized treatment strategies.
High myopia and pathological complications
High myopia, particularly pervasive in urban Asian regions, has increased from under 10% to between 10% and 20% within a decade. This condition carries substantial risks for the development of severe ocular pathologies, including myopic macular degeneration, cataracts, and retinal detachment, all of which can lead to blindness. For example, the incidence of retinal detachment is significantly elevated in individuals with high myopia. Efforts to mitigate the progression from low to high myopia are crucial in preventing these severe manifestations and related ocular complications.
High myopia is increasingly recognized as a significant public health concern due to its association with a spectrum of pathological ocular complications that can lead to irreversible vision impairment. The primary characteristics of high myopia include excessive axial elongation, which predisposes individuals to a range of structural and functional anomalies in the eye.85–87
Myopic maculopathy is a critical complication of high myopia, and its progression is notably correlated with the presence of posterior staphyloma (PS). Research indicates that eyes with posterior staphyloma are significantly more prone to the progression of myopic maculopathy, with a threefold increased likelihood compared to those without PS.85 The intricate structural deformities associated with wide macular posterior staphyloma, particularly its compound forms, exacerbate the severity of myopic maculopathy, resulting in poor visual acuity and greater axial elongation.88 Additionally, morphological studies using optical coherence tomography (OCT) reveal that PS significantly influences fundus morphological characteristics, with variations in the curvature of the sclera potentially impacting the development of related retinal pathologies.89
The continuous elongation of the axial length in high myopia patients is intricately linked to the severity of myopic maculopathy, particularly through its relationship with choroidal thinning.90 Choroidal thickness is a critical factor, with severe myopic maculopathy exhibiting notably reduced choroidal thickness compared to milder forms. This anatomical relationship underscores the potential role of choroidal degeneration in the pathophysiology of progressive myopic visual deterioration.
The relationship between high myopia and glaucoma, especially primary open-angle glaucoma, is well-documented, with increasing myopia severity correlating non-linearly with elevated glaucoma risk.86,87 The thinning of the retinal nerve fiber layer, detected via OCT, often precedes functional loss, suggesting that structural changes in highly myopic individuals warrant early detection and intervention strategies to preserve visual function. Furthermore, morphological changes in the optic nerve, such as defects in the lamina cribrosa, may complicate early glaucoma detection, necessitating comprehensive and regular examinations.87
The dome-shaped macula (DSM) phenotype in high myopia presents unique microcirculatory characteristics that are significantly distinct from those without DSM. Variations in the microvasculature, including reduced choroidal perfusion, are associated with DSM and contribute to variations in clinical outcomes.91 The role of structural parameters, such as scleral thickness and foveal choroidal perfusion, in the development and severity of DSM signifies an area ripe for further exploration, with potential implications for therapeutic interventions aimed at ameliorating blood perfusion issues in high myopia.
The models used for myopia research
In the pursuit of elucidating the mechanisms underlying myopia, animal models have played a pivotal role in advancing our understanding of this complex refractive error.92 Among the most widely utilized animal models are those involving form-deprivation and lens-induced myopia, frequently conducted in species such as chicks, mice, guinea pigs, and tree shrews.93 These models are invaluable due to their ability to mimic critical aspects of human myopia, facilitating the investigation of genetic, optical, and environmental influences on ocular development.
Chickens, in particular, have been extensively used in myopia research due to the rapid eye growth observed during their early development, which allows for timely experimental manipulation and observation.94 The chick model is instrumental in studying the effects of lens-induced alterations in focal plane positioning and subsequent compensatory ocular responses. Similarly, guinea pigs and tree shrews offer mammalian models with ocular physiology more akin to humans, enabling the exploration of myopic progression within a framework that closely parallels human eye growth patterns.95–97
Moreover, murine models, such as mice, are genetically tractable, providing opportunities for exploring the genetic underpinnings of myopia.98 The implementation of gene knockout and transgenic techniques in mice has greatly expanded the scope for investigating the genetic and molecular pathways implicated in myopia development.
By employing these animal models, researchers are not only able to simulate environmental conditions that influence myopia onset but also test potential pharmacological interventions, thus paving the way for translational advances in myopia management.
In summary, myopia presents a growing global public health concern, with epidemiological projections indicating a significant rise in affected individuals. This upsurge in myopia prevalence, particularly in urbanized regions of East and Southeast Asia, aligns with substantial variations across different geographic areas. Factors contributing to this variation include lifestyle choices such as extensive near work and reduced outdoor activities, in addition to socioeconomic and educational influences. Genetic predispositions also play a crucial role, with numerous studies identifying specific genes related to myopia development, emphasizing complex gene-environment interactions. A notable increase in high myopia, particularly among younger populations, underscores the urgency to address potential pathological complications, including myopic maculopathy and retinal detachment. This condition’s multifactorial nature requires a comprehensive understanding of genetic, environmental, and lifestyle determinants to formulate effective strategies for prevention and management. Highlighting the importance of early intervention, public health measures must prioritize education and behavioral adjustments to mitigate the adverse effects of myopia progression. Moreover, animal models continue to be instrumental in refining our understanding of the underlying mechanisms, contributing to translational efforts aimed at addressing this visual epidemic.
Diagnostic approaches in myopia
The diagnosis of myopia is crucial for effective management strategies aimed at mitigating its progression and effects. Traditional diagnostic approaches include subjective and objective refraction assessments, visual acuity tests, and comprehensive ocular examinations. Subjective refraction relies on patient interaction, typically using a phoropter or trial lens set, to determine the optimal lens power for visual clarity. In contrast, objective refraction employs autorefractors or retinoscopes to assess refractive error without patient input, providing an automated and precise measurement.99
Visual acuity tests, fundamental for myopia screening, often utilize the Snellen chart to evaluate a patient’s ability to discern letters or symbols at a set distance. The Early Treatment Diabetic Retinopathy Study chart offers an alternative, using the log of the minimal angle of resolution principle, which is favored in both research and clinical settings for its precision. Standard visual acuity is recorded in various notations, such as 6/6 in meters or 20/20 in feet, indicating normal distance vision. Myopia severity is frequently categorized, with mild myopia ranging from −0.50 to −3.00 D, moderate from −3.00 to −6.00 D, and high myopia beginning at −6.00 D.100
Comprehensive ocular examinations involve assessing both anterior and posterior segments through slit-lamp biomicroscopy and fundoscopy. This helps detect structural anomalies and potential complications, such as retinal detachment or myopic maculopathy, associated with high myopia.
Advances in diagnostic technologies
Recent advancements have significantly improved the precision and understanding of myopia diagnostics. OCT stands out as a pivotal tool, providing high-resolution cross-sectional imaging of the retina.101 It is essential for identifying retinal changes such as macular degeneration and vitreoretinal interface abnormalities commonly associated with myopia.
Optical biometry, utilizing technologies like partial coherence interferometry and swept-source OCT, facilitates precise axial length measurements, a critical factor in understanding myopia progression. These precise measurements are invaluable not only for diagnostics but also for evaluating therapeutic interventions aimed at slowing myopic progression.102
Further technological advancements, such as corneal topography and tomography, have enhanced the understanding of the corneal contribution to refractive error, offering detailed mapping of corneal shape and thickness.103 These techniques are particularly useful for assessing candidates for corneal refractive surgeries as a treatment option for myopia. Wavefront aberrometry, another emerging technology, provides comprehensive analyses of aberrations in the eye, contributing to a better understanding of visual quality in myopic patients.
School-based screening and pathological myopia
Early detection of myopia is crucial for preventing its progression to high myopia, which increases the risk of significant visual impairment. School-based screening programs, which predominantly use uncorrected visual acuity tests combined with non-cycloplegic autorefraction, enable early identification and management of myopic children. These programs help detect myopia in school-age children with high sensitivity and specificity.
Pathological myopia, a major cause of reduced visual acuity, especially in East Asia, necessitates careful fundus examination to confirm and assess myopic pathologies.6 Fundus photography, while useful, may require enhancements such as OCT or ultra-wide-field imaging for more accurate lesion detection and characterization. The META-PM classification system offers a detailed categorization of myopic maculopathy, aiding in the accurate staging and diagnosis of this severe form of myopia.6
The landscape of myopia diagnostics is robust, combining traditional methods with advanced technologies to enhance diagnostic precision and provide insights into the pathophysiology of myopia. These integrated approaches are pivotal for optimizing patient outcomes in myopia management, underscoring the importance of both classic and innovative tools in clinical practice.
In summary, the diagnosis of myopia involves a blend of traditional and advanced technological approaches, essential for effective management and mitigation of its progression. Traditional diagnostic methods encompass subjective and objective refraction, visual acuity tests, and thorough ocular examinations. Subjective refraction depends on patient feedback to determine corrective lens power, while objective refraction utilizes instruments like autorefractors for automated precision. Visual assessments, using charts like Snellen or the Early Treatment Diabetic Retinopathy Study, provide a baseline for evaluating the severity of myopia, which is clinically categorized by the degree of diopter deviation. Comprehensive examinations assess ocular health, detecting potential complications such as retinal detachment. Advances in technology have enriched myopia diagnostics, including Optical Coherence Tomography for detailed retinal imaging and optical biometry for precise axial length measurements, critical for understanding myopia progression. Emerging techniques like corneal topography and wavefront aberrometry offer detailed insights into corneal and visual quality, respectively. School-based screening programs emphasize early detection, while sophisticated methods like OCT enhance the evaluation of pathological myopia. Overall, these diagnostic advancements are crucial for optimizing patient management strategies and understanding myopia’s underlying mechanisms.
Interventional strategies for myopia management
Myopia, a prevalent refractive error, necessitates a multifaceted approach for its management and control. Interventional strategies encompass optical, pharmacological, lifestyle, and surgical options. Each strategy targets different aspects of myopia progression, requiring a personalized approach for effective management.
Traditional single-vision spectacles
Optical solutions are the primary line of defense against myopia and include glasses and contact lenses tailored for both corrective and control purposes. Traditional single-vision spectacles correct refractive errors and improve visual acuity but do not address myopia progression associated with axial elongation of the eye. Studies demonstrate that under-correction can worsen myopia progression by up to 30%.104,105 Thus, full correction remains essential for immediate visual correction, even if it does not slow progression.
Peripheral defocus spectacles
Peripheral defocus spectacle lenses have the potential to slow myopia progression by modulating the visual stimuli received by the peripheral retina. These lenses, through various designs and optical strategies, demonstrate significant potential in controlling myopia progression in children. Despite varying efficacies and the need for customization, evidence supports their use as integral components of myopia management strategies.
A comprehensive meta-analysis consolidated findings from several trials, affirming that peripheral defocus lenses significantly delay myopia progression compared to single-vision lenses (SVLs), though they did not show significant control over axial length growth. The enhanced myopia control effect suggests a distinct advantage for peripheral defocus lenses, with meta-analytic comparisons confirming their statistical superiority in controlling myopia progression.106
A study by Radhakrishnan et al. explored two designs of multiple-segment lenses—MiyoSmart and Stellest—and their impact on myopia progression over two years. The findings suggested that while initial efficacy was high, it declined over time but remained substantial compared to single-vision corrections. These lenses leverage asymmetrical through-focus image changes, leading to potential control through peripheral myopic defocus.107 The efficacy appears to reflect an absolute, rather than a proportional, mechanism of action. A study in Spain assessed the short-term efficacy of myopic peripheral defocus lenses, which demonstrated significant potential in slowing myopia progression over a one-year period. Children wearing myopic peripheral defocus lenses exhibited a 39% reduction in absolute axial length growth compared to the SVL group, marking a statistically significant advancement in myopia control.108 These results underscore the importance of optical designs in varying axial elongation rates. Zhang et al. explored the role of baseline relative peripheral refraction in influencing myopia control efficacy in children using Defocus Incorporated Multiple Segments (DIMS) lenses. The study concluded that individuals with baseline hyperopic relative peripheral refraction benefited more from myopia control, indicating the significance of customizing myopic defocus based on individual peripheral refractive profiles.109 This finding emphasizes that tailored optical interventions could optimize control strategies. Furthermore, a randomized clinical trial demonstrated that spectacle lenses with highly aspherical lenslets (HAL) and slightly aspherical lenslets significantly reduced the rate of myopia progression and axial elongation over a two-year period compared to SVLs. HAL was found to be more effective than slightly aspherical lenslets, with greater efficacy observed in children who wore HAL for at least 12 h daily. These findings suggest potential benefits of aspherical lenslets in controlling myopia progression.104
Multifocal soft contact lenses
In recent years, multifocal soft contact lenses have garnered attention as a promising approach for controlling myopia progression in younger populations. Various studies, including those reviewed by the American Academy of Ophthalmology, underscore the efficacy of these lenses.110 A comparative analysis of multifocal lenses versus single-vision spectacles or contact lenses revealed a consistent reduction in myopic progression. Specifically, the changes in SE ranged from 0.22 to 0.81 D across treatment groups compared to 0.50 to 1.45 D in control cohorts over at least one year.110 Additionally, axial elongation showed a similar trend, with a notable decrease in treatment groups. A meta-analysis further supported these findings, highlighting significant reductions in refraction progression and axial elongation in children using peripheral-add multifocal soft contact lenses compared to controls.111 The randomized, double-masked clinical trial conducted by Chamberlain et al. over a three-year period established the efficacy of MiSight daily disposable soft contact lenses in significantly reducing myopia progression among children aged eight to twelve years. The study demonstrated a 59% reduction in spherical equivalent refractive error and a 52% reduction in axial length growth compared to the control group, with no serious ocular adverse events reported. These findings underscore the potential of MiSight lenses as a viable intervention for myopia control.112 A randomized controlled trial demonstrated that DIMS spectacle lenses significantly slowed myopia progression and axial elongation in children compared to single-vision lenses. Over two years, children in the DIMS group exhibited 52% slower myopia progression and 62% less axial elongation, indicating the efficacy of DIMS lenses in providing clear vision with constant myopic defocus.113 These outcomes point to the ability of multifocal lenses to curtail natural myopic progression and warrant their inclusion in myopia management strategies.
The conceptual foundation for multifocal lenses lies in the manipulation of peripheral vision to prevent axial elongation. Research has shown that peripheral myopic defocus can potentially prevent the elongation associated with hyperopic defocus.114 Dual-focus contact lenses, by imposing a secondary focal plane, elicit modified central retinal responses, a key factor in halting the advancement of myopia.115 Multifocal lenses have demonstrated a propensity to modify these electrophysiological responses, particularly within the central 10° of the retina.115
Safety remains a paramount concern in evaluating new treatment modalities for children. Previous studies have reported minimal adverse events associated with the use of multifocal soft contact lenses.110 A meta-analysis documented an incidence rate of 0.065 for contact lens-related adverse events,111 indicating a favorable safety profile. Randomized controlled trials also affirmed the safety of the lenses, with most side effects being transient, such as dizziness and minor visual disturbances.114
The DIMS spectacle lenses, parallel to multifocal lenses, have yielded significant results in slowing myopia progression.116 A study encompassing a diverse demographic concluded the efficacy of these lenses in clinical practice, providing a benchmark for multifocal lens designs. Conversely, MiSight 1 day lenses have shown clinical success in inhibiting myopia progression among Korean children, supporting their use when orthokeratology lenses are unsuitable.117
While existing evidence supports the reduced progression of myopia with multifocal contact lenses, questions remain concerning optimal duration and effects post-treatment discontinuation.110 Further research, including diverse populations and real-world settings, is essential to validate the generalizability of these findings.114 Observational studies offer a prospective avenue for mitigating biases associated with industry-funded research and should be prioritized in future endeavors.114
Multifocal soft contact lenses emerge as a robust, safe, and effective option to address the burgeoning rates of childhood myopia. They represent a superior alternative to traditional single-vision corrections, thanks to their unique design that manipulates peripheral vision, thus slowing down myopia progression.
Orthokeratology
Orthokeratology has emerged as a significant intervention for myopia control, offering both inhibition of axial length elongation and reshaping of the cornea in myopic children. This review synthesizes findings from recent studies to provide a cohesive understanding of the efficacy, mechanisms, safety, and predictors of orthokeratology in myopia control.
Orthokeratology lenses have been proven effective in slowing axial elongation in children with myopia. A systematic review found that smaller back optical zone diameters in orthokeratology lenses resulted in more significant reductions in axial length growth compared to larger back optical zone diameters, highlighting the importance of lens design in therapeutic outcomes.118 A three-year follow-up study conducted in Scandinavian children also demonstrated consistent efficacy, regardless of the age at which treatment was initiated.119 Furthermore, a study comparing different orthokeratology lens designs revealed that those with a vision shaping treatment design had a more favorable impact on retarding axial length elongation compared to traditional corneal refractive therapy designs.120
The mechanism by which orthokeratology lenses inhibit myopia progression involves reshaping the cornea and modulating peripheral myopic defocus. Peripheral eye length evaluation using MRI showed that orthokeratology lenses led to a significant reduction in central axial length, with peripheral elongation occurring only beyond certain angles.121 Changes in choroidal vasculature and increased choroidal thickness have been observed during orthokeratology treatment, suggesting that these vascular changes may play a role in regulating ocular elongation.122 Dynamic modifications to corneal curvature and the e-value were correlated with control over axial elongation, as greater changes in corneal shape were associated with smaller increases in axial length.123
Predicting the long-term efficacy of orthokeratology lens correction involves evaluating baseline characteristics. Short-term efficacy has been shown to be a strong predictor of long-term therapeutic success, with age and baseline axial length serving as significant determinants.124 A crossover study further corroborated the stable efficacy of orthokeratology over periods extending up to three years, demonstrating its reliability as a long-term intervention.119
The safety profile of orthokeratology lenses, particularly concerning the potential for adverse events, has been a major consideration in their prescription for children. A pooled analysis of safety data showed that while adverse events did occur in a minority of orthokeratology wearers, these were mostly minor, including corneal abrasion and staining, with no serious adverse events reported.125 Compliance with lens maintenance protocols significantly mitigates these risks, making orthokeratology a viable myopia control method when appropriately managed.126
When compared to alternative methods, such as SVLs and HAL, orthokeratology exhibited superior control over axial length growth, although HAL showed slightly better outcomes in managing low myopia.127 Additionally, orthokeratology lenses enhance vision-related quality of life by providing spectacle-free daytime vision, making them a beneficial alternative to conventional corrective measures.126
Orthokeratology lenses offer a robust approach for managing myopia progression in children, supported by evidence of efficacy in controlling axial elongation, reshaping the cornea, and elucidating potential mechanisms at the vascular level. While safety remains a critical consideration, proper compliance significantly reduces risks, underscoring orthokeratology’s growing prominence as an intervention strategy.
Atropine eye drops for myopia
Pharmacological approaches are increasingly being explored, with atropine standing out as the most common agent. Atropine eye drops, particularly at low doses (0.01–0.05%), have gained considerable attention due to their efficacy in slowing eye growth and myopic progression.128 Evidence suggests that atropine, a muscarinic antagonist, positively impacts refractive error and AL in myopic children across various concentrations and treatment regimens. This review synthesizes findings from several key studies evaluating atropine’s effectiveness as a monotherapy and in combination with optical interventions.
Multiple studies underscore atropine’s efficacy in mitigating myopic progression across various concentrations. A network meta-analysis involving 5,422 eyes demonstrated that atropine concentrations such as 0.01%, 0.02%, and 0.05% significantly reduced progression rates in axial elongation compared to placebo, with the highest efficacy observed at 1%, albeit at the cost of increased risks, such as photophobia.128–129 A systematic review corroborated these findings, indicating that concentrations of 0.01% and higher are beneficial in myopia control, resulting in favorable changes in spherical equivalent refraction and AL.129 Notably, 0.01% atropine demonstrated moderate efficacy in reducing myopia progression without significant adverse effects, making it a preferred choice for low-dose treatments.130
Studies have also highlighted the comparative efficacy of atropine in conjunction with optical treatments. A comparative study involving 387 children evaluated 0.02% atropine against peripheral myopic defocus design spectacle lenses and orthokeratology.131 Results indicated that while orthokeratology showed superior control over axial elongation, 0.02% atropine still offered notable benefits, particularly in younger children. Furthermore, combination therapies, such as 0.01% atropine with orthokeratology or DIMS, showed enhanced control over spherical equivalent refraction and AL, suggesting a synergistic effect.132 The integration of 0.01% atropine with orthokeratology lenses was further evaluated in observational studies,133 which illustrated that the addition of atropine could significantly reduce AL elongation in fast-progressing myopic children compared to orthokeratology lenses alone. This combination approach optimized therapeutic outcomes, addressing individual variability in response to monotherapy.
Long-term studies provide insights into the durability of atropine’s effects and potential rebound phenomena post-treatment cessation. The Low-Concentration Atropine for Myopia Progression (LAMP) study, conducted over five years, revealed that low-concentration atropine, with a follow-up pro re nata regimen, sustained myopia control, with the highest concentration of 0.05% demonstrating continued efficacy.134 However, a randomized controlled trial identified a significant rebound in myopic progression after the discontinuation of 0.01% atropine, underscoring the need for continuous monitoring and potential re-treatment.135
The tolerability of atropine at low concentrations remains a critical factor in its recommendation for pediatric myopia management. A Danish trial reported that 0.01% atropine was well-tolerated over two years, with manageable adverse events.136 Enhanced pupil dilation and photophobia risks were notably higher at higher concentrations,129 suggesting the necessity for judicious selection based on risk-benefit assessments.
The LAMP study demonstrated that daily administration of 0.05%, 0.025%, and 0.01% atropine eye drops significantly reduced myopia progression compared to placebo, with a concentration-dependent effect evident after one year. The 0.05% concentration was the most effective in controlling spherical equivalent changes and axial length elongation, without negatively impacting visual acuity or quality of life. These results support the use of low-concentration atropine, particularly at 0.05%, as a viable option for myopia control in children.137 The two-year LAMP study demonstrated that 0.05% atropine eye drops maintained superior efficacy in reducing myopia progression compared to 0.025% and 0.01%, with efficacy twice that of the lowest concentration. Switching the placebo group to 0.05% atropine significantly reduced progression rates, and all concentrations were well-tolerated without detrimental effects on visual acuity or quality of life. These findings affirm 0.05% atropine as the most effective and optimal concentration for long-term myopia control.138 In the third year of the LAMP study, continued treatment with low-concentration atropine, particularly 0.05%, demonstrated superior efficacy in slowing myopia progression compared to a washout regimen, with significantly less progression in spherical equivalent and axial length. Although a concentration-dependent rebound effect was observed upon cessation, the clinical impact was minimal. Continuing treatment or stopping at an older age and lower concentration reduced rebound effects, reinforcing 0.05% atropine as the optimal choice for sustained myopia control over three years.139
Atropine eye drops, particularly at low concentrations such as 0.01% and 0.05%, are effective and generally safe options for controlling myopic progression in children. While high concentrations offer robust efficacy, they come with increased risk profiles. Integrating atropine with optical treatments such as DIMS and orthokeratology can optimize therapeutic outcomes, although careful consideration is needed for potential rebound effects post-treatment.
Lifestyle modifications
Emerging research underscores the impact of lifestyle on myopia development. Increased near-work activities are correlated with the onset of myopia, while outdoor activities help protect against its progression.140 Exposure to natural light and engagement in distance viewing can mitigate progression, promoting healthier eye development.141
Parental and educational interventions encourage balanced visual activities and sufficient outdoor time. These lifestyle modifications are integral to comprehensive myopia management, particularly as the global prevalence of myopia continues to rise.
Surgical options
Surgical interventions for myopia have evolved significantly, offering a variety of procedures tailored to different patient needs and clinical profiles. Among the most prominent techniques is laser-assisted in situ keratomileusis (LASIK), which reshapes the corneal stroma using an excimer laser to correct refractive errors.142 LASIK is favored for its rapid visual recovery and accuracy, although it requires adequate corneal thickness. Another innovative procedure is small incision lenticule extraction (SMILE), which utilizes a femtosecond laser to create a lenticule that is then removed through a small incision.143 SMILE is noted for its minimally invasive nature and potential to reduce dry eye symptoms while maintaining corneal biomechanics. For patients with higher degrees of myopia or thinner corneas, implantable collamer lenses provide an alternative. Implantable collamer lenses are inserted behind the iris and in front of the natural lens, providing reversible correction without altering the corneal structure.144 These surgical options collectively expand the therapeutic armamentarium for myopia, each with specific indications, benefits, and potential risks, enabling personalized patient care. Each surgical intervention involves specific risks and benefits, requiring detailed preoperative evaluations and discussions between the patient and ophthalmologist to ensure informed decision-making.
Others
The study by Jiang et al. evaluates the efficacy of repeated low-level red-light therapy for myopia control in a pediatric population through a multicenter randomized controlled trial. The findings suggest a significant reduction in myopia progression among children receiving this intervention compared to the control group. These results indicate potential therapeutic benefits and warrant further investigation into its long-term impact on myopia management.145
In summary, myopia management requires a comprehensive, multidisciplinary approach that integrates diverse interventional strategies. Optical interventions, such as peripheral defocus spectacle lenses and multifocal soft contact lenses, modify peripheral visual stimuli, thus mitigating progression and elongation rates. Orthokeratology demonstrates efficacy by reshaping the cornea and influencing peripheral defocus, yielding significant results in controlling axial elongation. Pharmacologically, low-dose atropine eyedrops, particularly in concentrations of 0.01% to 0.05%, exhibit effectiveness in slowing myopic progression, with reduced adverse effects compared to higher concentrations. Combining atropine with optical aids may enhance overall outcomes. Furthermore, lifestyle modifications, emphasizing increased outdoor exposure and balanced visual activities, are fundamental in comprehensive myopia management, given their protective effects against progression. Surgical options, though not first-line treatments, provide viable corrective solutions for refractory cases, with advances such as LASIK, SMILE, and implantable collamer lenses offering tailored patient care options. Emerging modalities, like repeated low-level red-light therapy, also show promise, necessitating further exploration. This integrative framework highlights the necessity of personalized strategies to effectively address pediatric myopia on a global scale.
Conclusions
Myopia represents an escalating global public health challenge, significantly influenced by genetic predispositions and lifestyle factors such as extensive near work and limited outdoor activities, particularly in urbanized areas of East and Southeast Asia. Comprehensive diagnostic techniques, ranging from traditional methods to advanced technologies like Optical Coherence Tomography, are vital in effectively detecting and managing myopia. A multidisciplinary management approach—including optical, pharmacological, and lifestyle interventions—facilitates the mitigation of myopia progression and related complications. Future efforts should prioritize early intervention strategies and further investigate emerging therapeutic modalities, ensuring a nuanced understanding of the multifactorial nature of myopia to devise effective prevention and management frameworks.
Declarations
Acknowledgement
We extend our sincere gratitude to Dr. Andrea Sonntag from the Department of Ophthalmology at Parkway Gleneagles Medical and Surgical Center, Shanghai, China, for her invaluable insights and assistance in shaping this review.
Funding
None.
Conflict of interest
One of the authors, Peng Zhou, has served as an Executive Associate Editor for Nature Cell and Science since January 2024. The authors declare no other conflicts of interest.
Authors’ contributions
Literature review, draft of the manuscript (ZP, DW), and collection of literature (DW).

 Author information
Author information