Introduction
Aging encompasses the progressive deterioration of bodily functions over time, driven inexorably by the confluence of various pathological, physiological, and psychological processes.1 Aging leads to increased susceptibility to disease, reduced functional reserve, and decreased healing and stress resistance, which makes health unstable.2 Aging of the body can lead to a variety of metabolic diseases, including cardiovascular disease, obesity, insulin resistance, non-alcoholic fatty liver disease (NAFLD), chronic hepatitis, and osteoporosis. In recent times, there has been a gradual unveiling of the molecular mechanisms connecting chronic liver disease to osteoporosis. Different types of chronic liver diseases lead to different mechanisms of osteoporosis, and the liver-bone axis is a pivotal focus of research. Focusing on common chronic liver diseases such as NAFLD, cholestatic liver disease, and viral hepatitis, this review summarizes the mechanisms of different chronic liver diseases leading to osteoporosis in recent years.
Aging and liver
As the largest glandular organ in the human body, the liver maintains major metabolic processes in the body, such as toxin metabolism, inflammation regulation, and synthesis of various biomolecules involved in important physiological activities.3 With advancing age, the liver undergoes degenerative changes in shape and structure, diminishing in size and regenerative capacity compared to its youthful state.4–6 During aging, the liver tends to exhibit large hepatocytes and polyploidy. There is an increase in nuclei and nucleoli, as well as ultrastructural changes in liver organelles. Moreover, considerable heterogeneity is often observed among different cells, even within the same lobule.7
Importantly, aging is a significant risk factor for various liver diseases, such as hepatitis, liver fibrosis, cirrhosis, and hepatocellular carcinoma. Additionally, the incidence of chronic liver disease increases with age and is associated with a poorer prognosis.8,9
Immune imbalances during aging result in continuous proinflammatory factor release, termed inflamm-aging, contributing to liver dysfunction.10,11 The markers of liver necrosis rise with the increase of inflammatory factors, and anti-inflammatory treatment can also alleviate the aging of liver.12 In addition, SIRT2 is closely related to aging and involved in the regulation of inflammation, with increased expression observed in senescent hepatocytes, playing an important role in aging liver inflammation. Chronic inflammation increases the risk of various chronic liver diseases.
Liver fibrosis is a common occurrence in most chronic liver diseases. The accumulation of inflammatory-activated hepatic stellate cells (HSCs) in the liver contributes to liver fibrosis, serving as a precursor to liver cirrhosis.13 Initially, HSCs are activated and undergo proliferation in response to chronic liver injury, leading to their deposition in the extracellular matrix of fibrotic scars. Studies have shown that in mice lacking regulatory effects on senescence factors, HSCs continue to proliferate, eventually resulting in excessive liver fibrosis.14 Senescent HSCs can promote the resolution of fibrosis, but the senescence of hepatocytes and choanocytes can also contribute to the development of liver fibrosis.15 Liver fibrosis in patients with chronic liver disease is closely associated with aging. Cirrhosis frequently develops in the advanced stages of liver fibrosis and is prevalent among elderly patients.16
Senescence is one of the significant factors contributing to chronic liver diseases. Senescent hepatocytes can induce senescence in neighboring hepatocytes, a phenomenon known as senescence-induced senescence. This process, in turn, continues to activate HSCs and eventually exacerbates liver fibrosis.17 The incidence of chronic liver disease increases with age, underscoring the pivotal role of liver metabolism and its implications for various complications.
Osteoporosis
Osteoporosis is a systemic disease characterized by the loss of bone mass, strength, and microstructure, leading to decreased bone strength.18 With the aging of the population, the incidence of osteoporosis is increasing annually.19 Osteoporosis is a significant health concern in aging individuals, as the gradual loss of bone mass during aging leads to osteopenia and osteoporosis.20 The prevalence of osteoporosis in elderly worldwide is as high as 35.3%.21 Notably, the risk of osteoporosis is significantly elevated in elderly women compared to elderly men, attributed to the declining estrogen levels and increased post-menopausal bone loss.22
Bone metabolism is primarily regulated through coordinated interactions among osteoblasts, osteoclasts, and osteocytes facilitated by key molecules to maintain normal bone formation.18 Under healthy conditions, osteoblasts secrete various factors to regulate bone metabolism, and the key cytokine regulating the transition between osteoblasts and osteoclasts is the receptor activator of NF-kB ligands (RANKL). By binding to the RANKL receptor on the surface of osteoclast precursor cells, RANKL induces their differentiation into mature osteoclasts.23 The RANKL-RANK axis plays a critical role in osteoclast differentiation and maturation. When bone resorption mediated by osteoclasts exceeds bone formation by osteoblasts, the normal balance of bone metabolism is disrupted, leading to increased bone loss—the foundation of osteoporosis pathogenesis.18 Additionally, the aging environment in the body can contribute to an increased production of osteoclasts and the generation of an inflammatory degenerative niche.24
The pathogenesis of osteoporosis is not solely attributed to the imbalance of cooperation among bone cells but is also closely linked to other organs and tissues.18 Bone metabolism is tightly regulated by various endocrine and signaling molecules throughout the body, including the effects of growth, nerves, and gonadotropin subclasses.25,26 As the metabolic center of the whole body, the liver plays a crucial role, and osteoporosis is a common complication in various chronic liver diseases. Notably, 40% of patients with chronic liver disease have secondary osteoporosis,27 indicating a significant relationship between the liver and bone.
Liver-regulatory role and mechanism of bone metabolism
Recent studies have illuminated the potential role of the liver in influencing bone metabolism through diverse mechanisms, encompassing exosome delivery, hormonal regulation, pro-inflammatory factor modulation, and protein modification. In this review, we classify different aspects of the molecules that affect bone metabolism, including liver-related metabolic factors, cytokines, and proteins involved in the regulation of bone metabolism, and focus on the effects of exosome secretion on bone metabolism. Simultaneously, we delve into additional potential influences tied to chronic liver diseases. The purpose of this paper is to provide readers with a more comprehensive introduction to the molecules involved in liver-to-bone communication.
Role of hepatic metabolism factors in bone metabolism
VitaminD3 (VitD3)
In the body, VitD3 is primarily synthesized in the skin. VitD3 is produced from 7-dehydrocholesterol (7-DHC) upon exposure to ultraviolet irradiation,28 and a small amount is absorbed by the intestines.29 Hepatic enzymes, namely, vitamin D25 hydroxylase, catalyze the hydroxylation of VitD3, transforming it into its active form 1,25-dihydroxyvitamin D3 (1,25-(OH)2-VitD3).30,31 This active form plays a crucial role in maintaining healthy bones. DHC, a crucial precursor of VitD3, is synthesized from cholesterol in the liver by cholesterol 7α-hydroxylase (CYP7A1) and subsequently degraded to cholesterol by 7-DHC reductase (DHCR7).32,33 Increased levels of DHCR7 have been found to contribute to the degradation of 7-DHC, a factor linked to the development of osteoporosis.32,33 Although the activity and quantity of 1,25(OH)2D are not affected in patients with cirrhosis caused by cholestatic liver disease,34,35 bone loss persists,36 with the elevated content of DHCR7 potentially playing a contributing role. In addition, CYP2R1 and CYP27A1 exhibit decreased expression in the cirrhotic liver, leading to the levels of 1,25(OH)2D products, namely, 24,25-dihydroxyvitamin D (24,25(OH)2D) and 1,24,25-trihydroxyvitamin D (1,24,25(OH)3D3), are increased in cirrhosis patients, indicating diminished activity of VitD3 in individuals with chronic liver disease.
In conclusion, the role of VitD3 in preventing osteoporosis in patients with liver cirrhosis is complex and needs further study.
Fibroblast growth factor 21 (FGF-21)
FGF-21 is a metabolic hormone secreted by the liver that functions as a regulator of glucose and lipid metabolism.37 It has been identified as a negative regulator of bone homeostasis.38
Serum FGF21 levels were found to be elevated in patients with non-alcoholic steatohepatitis (NASH)/NAFLD,39 indicating a response to liver lipid accumulation, and serving as a biomarker.40 Insulin-like growth binding protein (IGFBP1), an endocrine hormone from the liver, plays a crucial role in the regulation of osteoclastogenesis. Moreover, FGF21 can increase the secretion of IGFBP1, and IGFBP1, in turn, facilitates osteoclastogenesis by enhancing RANKL signaling through its receptor integrin β1.41 In mice undergoing ovariectomy surgery to simulate postmenopausal conditions in women, the administration of an IGFBP1 inhibitor blocked FGF21-enhanced bone resorption, significantly reduced serum FGF21 levels, and effectively alleviated bone loss.41
Recombinant human FGF21 (rhFGF21) is a potential drug for the treatment of NAFLD and type 2 diabetes, but the side effects of this drug on osteoporosis are worrisome.42 In future studies, IGFBP1 antagonists could be explored as a potential solution to address this issue.
Insulin-like growth factor-1 (IGF-1)
IGF-1 is a vital growth factor secreted by the liver.43 The liver is the primary site for IGF-1 secretion in the body, with a small amount also being secreted in bone.44 IGF-1 presents in ternary complexes, consisting of IGF molecule, IGF-binding protein 3 (IGFBP-3), and acid-labile subunits (ALS), serving as the principal storage form of IGF-1.45 Studies involving the targeted knockout of IGF-1 or ALS specifically in the liver of mice showed that while IGF-1 levels decreased to varying degrees, normal bone growth was still maintained.45 However, when both IGF-1 and ALS were knocked down, IGF-1 levels were significantly reduced, resulting in a substantial weakening of bone growth.45 These findings suggest that a certain amount of IGF-1, IGFBP-3, and ALS is necessary to maintain bone homeostasis.
In NASH/NAFLD patients, the serum level of IGF-1 was notably lower compared to the control group.43 The reduction of the IGF-1/IGFBP-3/ALS ternary complex may also contribute to the development of osteoporosis caused by chronic liver diseases.46 In conclusion, different IGF-1 and its complexes play unique roles in bone growth, exerting different effects on cortical and trabecular bone growth.47
Fetuin-A
Fetuin-A, also known as alpha 2-Heremans-Schmid glycoprotein (AHSG), is synthesized in the liver and plays a significant role in insulin resistance.48 Elevated levels of Fetuin-A are observed in NAFLD, and a notable improvement in NAFLD is noted when Fetuin-A is reduced through dietary and exercise interventions.49,50
Recent studies have indicated that the level of circulating Fetuin-A is associated with bone metabolism, serving both as a mineral carrier protein and an inhibitor of systemic extra-osseous calcification.51 In a study on Fetuin-A-deficient Ahsg−/− mice, femur growth was restricted.52 Similarly, postmenopausal women with osteoporosis due to inadequate estrogen secretion exhibit lower serum Fetuin-A levels than their peers.53 When pursuing fetuin-A targeting for the treatment of obesity or type 2 diabetes to maintain insulin sensitivity, it is crucial to acknowledge the potential risk of disrupting bone homeostasis.
Oncofetal fibronectin (OFN)
Fibronectin (FN) is a ubiquitous extracellular matrix protein in the body that plays a pivotal role in bone formation. It regulates osteoblast differentiation and promotes the recruitment of osteogenic precursor cells.54 In the absence of FN, fibroblasts can still differentiate into osteocytes, but these osteocytes are unable to undergo mineralization, thereby impacting bone formation.55 The glycosylation of FN at threonine residue 33 results in OFN.55
Patients with cholestatic liver disease often exhibit elevated levels of OFN.56 Researchers have noted a negative correlation between OFN and the bone formation marker osteocalcin, suggesting that increased OFN levels are associated with decreased bone formation.55 However, this correlation is specific to cholestatic liver disease and has not been observed in other chronic liver diseases.55
Under normal circumstances, the liver produces proto-plasma fibronectin (pFN).57 In patients with chronic liver disease, FN levels increase, and HSCs undergo glycosylation of FN to form OFN due to cytotoxins such as CCl4.57 Recent studies on OFN and osteoblasts have shown that glycosylation of OFN hinders the differentiation of osteoblasts, leading to hepatic osteodystrophy, but this harmful effect can be reduced by binding α4β1. Because OFN is a type of FN, FN can bind to various integrin pairs located on the cell surface through different domains, thereby influencing different intracellular signals in cells.56,58 The reduction in mediated signaling due to FN glycosylation may result in hepatic osteodystrophy. This decrease in signal transduction can potentially be counteracted by the binding of α4β1 integrin to antibodies or peptides, providing a novel strategy for the treatment of hepatic osteodystrophy.56
Role of modifying enzymes in liver-bone communication
SIRT2
SIRT2, a nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylase, is the primary silent regulatory protein highly expressed in the liver.59 SIRT2 plays a significant role in aging and metabolic regulation and is also involved in cell differentiation and apoptosis. In macrophages, SIRT2 deacetylates NLRP3, inactivating the NLRP3 inflammasome and offering a potential target for reversing aging-associated inflammation and insulin resistance.60 Moreover, studies have shown that SIRT2 is implicated in various liver diseases, such as alcoholic liver disease, NAFLD, and liver fibrosis.61,62
Recent research has revealed that hepatocyte SIRT2 also plays a crucial role in the liver-bone axis, maintaining bone homeostasis and preventing osteoporosis.63 This study showed that hepatocyte SIRT2 expression is increased in aged mice and older patients. Liver-specific SIRT2 deficiency (SIRT2-KOhep) obviously inhibits osteoclastogenesis and alleviates osteoporosis in aged and postmenopausal osteoporosis mouse models. Mechanistically, leucine-rich alpha-2-glycoprotein 1 (LRG1), which is required for the protection of SIRT2-KOhep against osteoclastogenesis, was identified as the functional cargo in hepatocyte-derived small extracellular vesicles (sEVs). In hepatocytes, SIRT2-KOhep up-regulates the expression of LRG1 in hepatocyte-derived sEVs (sEVs-LRG1) through increasing the acetylation of H4K16. The sEVs-LRG1 is transferred to bone marrow-derived monocytes (BMDMs) to suppress osteoclast differentiation by directly inhibiting the nuclear translocation of NF-κB p65. Therapeutically, treating ovariectomized mice with the SIRT2 pharmacological inhibitor AGK2 or sEVs purified from LRG1-overexpressing AML12 hepatocytes obviously attenuated osteoclastogenesis and bone loss. Accordingly, sEVs derived from human LRG1-high plasma or hepatocytes inhibited by SIRT2 may markedly inhibit human osteoclast differentiation. Moreover, treatment with high-expressing-LRG1-sEVs was superior to denosumab in preventing the rebound effect of human osteoclastogenesis. Importantly, the clinical data showed that the plasma sEVs-LRG1 concentration was positively correlated with bone mineral density and negatively related to bone resorption markers in patients. The regulation of sEVs-LRG1-BMDM-p65 axis by hepatic SIRT2 might lead to effective therapeutic targets for treating osteoporosis.63
Lecithin cholesterol acyltransferase (LACT)
LACT is the enzyme responsible for the formation of cholesterol esters from unesterified cholesterol (UC) and phospholipid (PL) molecules in high-density lipoprotein cholesterol particles.64 Protein phosphatase 2A (PP2A) is a critical member of the protein phosphatase family and plays a regulatory role in phosphorylation modifications in most eukaryotic cells.65 In addition, studies have shown that PP2A reduces CCl4-induced acute liver injury.66,67
The expression of PP2Aα was found to be significantly increased in the livers of patients with chronic hepatobiliary disease, leading to the downregulation of hepatocyte LCAT expression. In PP2Aα-deficient mice, osteoporosis was significantly improved, and LCAT expression in the liver was notably increased. Studies have revealed that PP2A regulates LCAT expression by dephosphorylating the transcription factor USF1, which contributes to the improvement of osteoporosis in mice.68
In addition, there is a positive correlation between the content of high-density lipoprotein and bone mass. Metabolically disordered high-density lipoprotein can impact normal bone formation in various ways.69 LCAT, due to its association with cholesterol transport,70 has been found to regulate the level of intracellular cholesterol. It plays a role in maintaining the balance of bone metabolism between osteoblasts and osteoclasts and promotes reverse cholesterol transport from bone to the liver, leading to the improvement of osteoporosis and the reduction of liver fibrosis in mice.68
Tumor necrosis factor-alpha (TNF-α)
TNF-α is an immunomodulatory proinflammatory factor primarily produced by macrophages, T cells, and NK cells.71 Its secretion is significantly increased in patients with glucose and lipid metabolism disorders, viral hepatitis, or NAFLD, affecting bone metabolism as well. TNF-α promotes chronic liver inflammation, exacerbates liver injury, and contributes to chronic hepatitis in NAFLD patients.72 Moreover, TNF-α exacerbates the inflammatory response in other parts of the body. TNF-α induced by osteoclasts participates in the processes of rheumatoid arthritis, orthopedic implant loosening, and other forms of chronic inflammatory osteolysis. It is closely related to RANKL, as TNF-α requires RANKL to regulate macrophages and stabilize osteoclasts.73 Furthermore, TNF-α can enhance osteoclast generation through macrophage colony-stimulating factor (M-CSF).74 However, TNF-α not only affects osteoclasts but also inhibits the recruitment and differentiation of progenitor cells to osteoblasts.75 Increased TNF-α levels are observed in estrogen-deficient mice.76 Additionally, a negative correlation exists between vitamin D and TNF-α levels.77 This suggests that TNF-α may play a role in the crosstalk between the liver and bone.
Colony-stimulating factor-1 (CSF-1)
CSF-1, also known as M-CSF, is a chemotactic factor for monocytes and macrophages.78 In cholestatic liver disease patients with osteoporosis, there is a significant increase in the number of osteoclast-like cells with evident formation of peripheral monocytes. The osteoclast-like cells exhibit functional activity when co-cultured with both CSF-1 and receptor activator of nuclear factor κβ ligand (RANKL) in vitro.79 This finding suggests that osteoporosis in cholestatic liver disease may be related to the early formation of osteoclasts and that the content of CSF-1 is increased in patients with cholestatic liver disease complicated with osteoporosis. CSF-1 may be an important key factor responsible for guiding monocytes to form osteoclasts79
Interleukin (IL)-17
IL-17 is a member of the inflammatory cytokine family. Together with T helper 17 (TH17) cells, IL-17 plays a crucial role in tissue inflammation, autoimmunity, and host defense.80,81
TH17 cells influence bone homeostasis by promoting the differentiation of osteoclasts.82,83 In autoimmune arthritis, a subset of TH17 cells with high levels of RANKL and pro-inflammatory factors is present, contributing to bone loss. IL-17, produced by TH17 cells, is closely associated with bone loss.84 Studies on alcoholic fatty liver disease have reported extensive infiltration of TH17 cells in the liver, leading to the production of IL-17.85 Elevated IL-17 in patients with alcoholic fatty liver disease may represent another pathway of liver-bone crosstalk.
Osteopontin (OPN)
OPN is a growth regulatory protein and factor that is widely expressed in various cells and tissues. Moreover, OPN plays an important role in the metabolic processes of organs and tissues, contributing to aging.86 In patients with NAFLD/NASH, OPN is abundantly expressed in the liver and activates hedgehog signaling, promoting liver fibrosis.87 Genetically, OPN-deficient mice exhibit reduced triglyceride synthesis, preventing obesity-induced hepatic steatosis.88
Notably, OPN is not only involved in liver metabolic diseases but also has an impact on bone metabolism, promoting bone loss in patients with osteoporosis. It acts as a downstream signaling molecule of RANK/RANKL,89 promoting osteoclast differentiation and proliferation. Moreover, OPN was demonstrated to interact with CD44, an essential component of osteoclast activity. It is suggested that OPN controls the migration and adhesion of osteoclasts to the bone matrix by enhancing CD44 expression on osteoclasts.90 OPN-deficient mice had resistance to osteoporosis after ovariectomy surgery, and osteopontin has been clinically identified as a high-risk factor for osteoporosis in postmenopausal women.91 Furthermore, subsequent studies have revealed that OPN leads to parathyroid hormone induced tartrate-resistant acid phosphatase positive cells increase in bone, which helps protect from osteoporosis through increasing bone resorption.92 Due to its involvement in bone metabolism, OPN represents a potential therapeutic target for hepatic osteodystrophy.
Osteoprotegerin (OPG)
OPG, a member of the tumor necrosis factor receptor superfamily, is a decoy receptor for tumor necrosis factor-related apoptosis-inducing ligands.93 In NASH patients, the concentration of OPG is significantly reduced.94 Initially, OPG was considered the factor that led to increased bone mineral density (BMD). However, clinical practice has revealed that OPG can affect the BMD of the lumbar spine in men but has no effect on the BMD of other organs.95 In females, OPG influences the BMD of the vertebrae.96 In general, the role of OPG in patients with chronic liver disease and osteoporosis still needs further study.
Conclusion
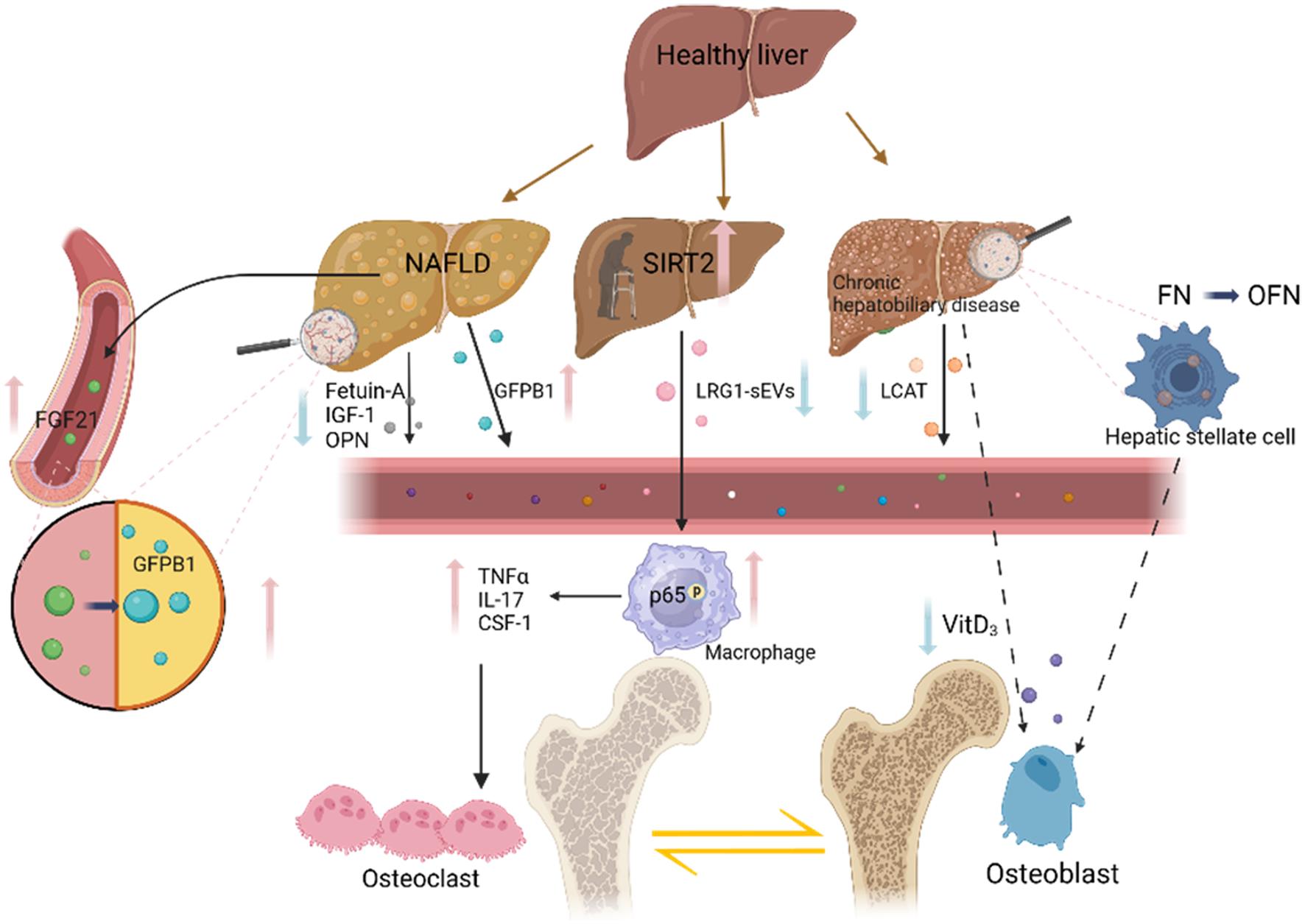
In the context of population aging, individuals often contend with a dual burden of morbidity and a diversity of age-related diseases. Nearly all patients with chronic liver disease undergo disruptions in bone metabolism, with up to two-thirds presenting signs of osteoporosis. This prevalence significantly compounds the challenges in treatment.68 While chronic liver disease and osteoporosis may seem unrelated among the elderly, numerous studies have investigated the ability of the liver to influence bone metabolism through various pathways. Consequently, unraveling the intricate relationship between the liver and bone is of paramount importance. The liver can influence the pattern of bone metabolism through a variety of mediators. Not only is Vitamin D3 closely associated with osteoporosis among hepatic metabolites, but also various hormones and growth factors secreted by the liver exert distinct impacts on bone metabolism. Factors such as FGF-21, IGF-1, IGFBP1, Fetuin-A, and pro-inflammatory factors like TNF-α, IL-17, and CSF-1 all play significant roles in the intricate process of bone metabolismFurthermore, modifying enzymes such as SIRT2 and LACT, along with OPN and OCN, play significant roles in bone remodeling. The impact of SIRT2 on bone homeostasis was found to influence the regulation of the RANK-RANKL axis through the trafficking of LRG1 to sEVs. On the other hand, when the level of FN-glycosylated OFN is increased in chronic liver disease, bone remodeling is reduced, and bone loss is increased. Most cytokines and proteins affect RANKL directly, influencing the differentiation and formation of osteoclasts and bone homeostasis. LCAT is suggested to maintain bone through intracellular cholesterol regulation, which supports the effect of lipid metabolism disorders on bone metabolism in chronic liver disease. Additionally, the occurrence and development of liver disease are closely linked with chronic inflammation induced by aging. Some inflammatory factors, such as TNF-α and IL-7 as mentioned above, can lead to bone loss. Pro-inflammatory factors have the potential to mediate liver-bone communication, although there is no direct evidence supporting this phenomenon (Fig. 1).
Solid black arrows indicate pathways affecting osteoclasts, while the dashed black arrows represent pathways affecting osteoblasts. FGF21 levels are elevated in the serum of NAFLD/NASH patients, leading to increased liver secretion of GFBP1 and the promotion of osteoclast generation. NAFLD patients exhibit elevated levels of Fetuin-A, IGF-1, and OPN, inducing osteoclast formation. Elevated SIRT2 levels in aged hepatocytes decrease the secretion of LRG1-sEVs and increase the phosphorylation of p65 in the nucleus of macrophages, contributing to bone loss. In chronic liver disease, the number of macrophages and pro-inflammatory- cytokines TNF-α, IL-17, and CSF-1 increase, enhancing bone metabolism. Decreased Vitamin D3 results in reduced osteoblast production. Oncoembryonic fibronectin produced by activated stellate cells in primary biliary cirrhosis patients inhibits osteoblasts and bone formation. At present, many studies on liver-bone communication are based primarily on rodent models, and their direct applicability to clinical settings is limited. Furthermore, the specific mechanisms related to the involvement of cytokines and proteins in liver-bone communication remain poorly understood. With the emergence of advanced multi-omics research in the current era, there are increasingly promising avenues for delving into the pathophysiological intricacies of liver-bone communication. This holds great potential for developing enhanced treatment strategies for chronic liver diseases accompanied by osteoporosis. [VitD3, vitaminD3; TNF-α, TNF alpha;FGF-21, Fibroblast growth factor 21; LACT,Lecithin cholesterol acyltransferase;FN, Fibronectin; OFN, Oncofetal fibronectin ; GFBP1, Insulin-like growth binding protein; IGF-1,Insulin-like growth CSF-1, factor-1; Colony-stimulating factor-1; LRG1,leucine-rich alpha-2-glycoprotein 1; OPN, Osteopontin; IL-17, Interleukin (IL)-17]. Created with BioRender.com.
Abbreviations
- CSF-1:
colony-stimulating factor-1
- FGF-21:
Fibroblast growth factor 21
- FN:
Fibronectin
- GFBP1:
Insulin-like growth binding protein
- IGF-1:
insulin-like growth factor-1
- IL-17:
Interleukin (IL)-17
- LACT:
Lecithin cholesterol acyltransferase
- LRG1:
leucine-rich alpha-2-glycoprotein 1
- OFN:
Oncofetal fibronectin
- OPG:
Osteoprotegerin
- OPN:
Osteopontin
- TNF-α:
TNF alpha
- VitD3:
vitaminD3
Declarations
Acknowledgement
None.
Funding
This study was funded by the National Natural Science Foundation of China (No.82070603, 32371244, 82270244, 82200654,92057118); Shanghai Natural Science Foundation (19ZR1428400); Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZDCX20212000).Guangxi Training Program for Medical High-level Academic Leaders (No. Guiweikejiaofa [2020]-15), Bose Talent Highland (No. 2020-3-2), Building Projects of Guangxi Bagui Scholars (No. 2024), Building Projects from the Key Laboratory of Molecular Pathology (for Hepatobiliary Diseases) of Guangxi (No. Guiweikejiaofa [2020]-17) and the Key Laboratory of Tumor Molecular Pathology of Guangxi Higher Education Institutes (Guijiaokeyan [No. 2022]-10), and Clinical Key Specialty Building Project (For Pathology) of Guangxi (No. Guiweiyifa [2022]-21).
Conflict of interest
The authors declare no competing interests.
Authors’ contributions
Contributed to study concept and design (MH), drafting of the manuscript (JL, YTZ), critical revision of the manuscript (YKF, YJH, HZZ, LCH, YHB and YH), supervision (FYK,XDL).All authors made a significant contribution to this study and have approved the final manuscript.

 Author information
Author information