Introduction
Age-related macular degeneration (AMD) is a disease that mainly affects the vision of the elderly and is one of the main causes of blindness in developed countries. With the increasing trend of population aging, the incidence of AMD rate and social burden are also increasing, and are expected to reach 288 million in 2040.1–3 Therefore, it is of significant clinical significance to deeply explore the pathogenesis of AMD and find effective treatment strategies.
AMD is a multifactorial disease involving complex interactions between aging, genetic predisposition, and environmental risk factors.4–7 Multiple reviews have shown that chronic inflammation, oxidative stress, and lipid deposition are closely related to the pathogenesis of AMD.8–12 The current study found that the accumulation of cellular waste and problems with its clearance also play an important role in its pathological mechanism.13–16 The ubiquitin-proteasome system (UPS) and autophagy are two important mechanisms for degrading and removing abnormal proteins and organelles in cells.17,18
The UPS delivers soluble, short-lived, misfolded, or damaged proteins to the proteasome, which has important functions in the regulation of cellular signaling and transcription.19,20 Autophagy is a vesicular trafficking pathway specifically responsible for the delivery of long-lived proteins, soluble or insoluble protein aggregates, and damaged organelles to lysosomes.21–23 There are complex interactions and regulatory mechanisms between UPS and autophagy. Selective autophagy requires UPS to participate in the ubiquitination and degradation of target proteins, and the function and synthesis of UPS are also affected by autophagy.17 However, in AMD, the separate or synergistic effects of ubiquitin-proteasome and autophagy are impaired, resulting in the accumulation of damaged organelles and abnormal proteins, which in turn trigger inflammatory responses and cell death, accelerating the progression of AMD.10,16,24
This review aims to systematically summarize and analyze the interaction and regulation of ubiquitin-proteasome and autophagy in the pathogenesis of AMD. We review the existing literature, explore the function and abnormality of ubiquitin-proteasome and autophagy in AMD, and analyze their association with AMD and possible therapeutic strategies. A deep understanding of the mechanism of ubiquitin-proteasome and autophagy is of great significance for revealing the pathogenesis of AMD and developing therapeutic strategies.
AMD
AMD is clinically classified as early and late stage depending on the size of the drusen and on pigmentary abnormalities.25 Vision impairment in early AMD is mildest and is often accompanied by reduced reading ability, vision distortions, and black or gray spots in central vision. In the late stage of AMD, large or moderate drusen or pigmentary abnormalities occur, and the patients’ central vision is significantly affected.26 Late AMD can occur in dry (atrophic) or wet (exudative, neovascular) forms.25 Dry AMD is the most common form of AMD, and it accounts for approximately 80–90% of AMD patients. It usually involves progressive atrophy of the retinal pigment epithelium (RPE), choroidal capillaries, and photoreceptors. Patients with dry AMD usually experience slow or limited vision loss.27,28 Neovascular AMD (nAMD) is a more rapidly progressive and severe form of AMD, accounting for approximately 10–20% of AMD cases. nAMD is primarily caused by the growth of abnormal new blood vessels that leak blood, lipids, and fluid into the macular area, causing damage and scarring of retinal tissue.29,30
AMD is a progressive, degenerative eye disease that affects the macula, the central area of the retina, and causes distorted vision. The hallmark of AMD is the accumulation of lysosomal lipofuscin in the RPE cells of the macula, the presence of extracellular drusens between the basal layer of the RPE and Bruch’s membrane, and the progressive degeneration of photoreceptors and adjacent tissues, leading to loss of central vision.31–33
Role of UPS in AMD
The UPS is a cytoplasmic protein degradation system involved in the ubiquitination and degradation of target proteins.34 The UPS is the major proteolytic pathway for short-lived, misfolded, and damaged proteins. It has important functions in the regulation of cellular signaling and transcription and is involved in a variety of cellular functions.35 The key molecule in this system, ubiquitin, is a small protein marker that, by binding to a target protein, marks it for degradation. The ubiquitination process involves the synergy of multiple enzymes such as ubiquitin-activating enzyme, ubiquitin-conjugating enzyme, and ubiquitin ligase (E3 ligase). Once a target protein is ubiquitinated, it is recognized and sent to the proteasome for degradation.34 There are a variety of proteases inside the proteasome, which can degrade ubiquitinated proteins into small molecules to maintain the dynamic balance of intracellular proteins.36,37 At present, some studies have found that the abnormality of UPS is also related to the occurrence and development of AMD.
Protein homeostasis regulation
The main function of the UPS is to clear abnormal or aged proteins in cells through ubiquitination and proteasomal degradation. Studies have found that the abnormality of the ubiquitination process, the defect of the E3 ligase, and the degradation of the protein in the proteasome are blocked, resulting in the accumulation and aggregation of abnormal proteins, causing cell damage and apoptosis, and then leading to AMD.38
Oxidative stress and inflammation
Impairment of the UPS may lead to increased intracellular oxidative stress and inflammatory responses. This is due to the inability of damaged UPS to effectively remove oxidatively damaged proteins and activated inflammatory mediators in a timely manner. The increase of oxidative stress and inflammatory reaction leads to further expansion of cell damage, thereby accelerating the progression of AMD.39
Genetic factors
Certain genes and genetic variants are associated with the risk of developing AMD. Some of these genes are key genes for UPS, and mutations in these genes cause the UPS to function abnormally, thereby increasing the risk of developing AMD in patients. In our previous study of the East Asian AMD population, we found that mutations in the ubiquitin protein ligase E3D (UBE3D) gene can lead to AMD. UBE3D accepts ubiquitin from specific E2 ubiquitin-conjugating enzymes and transfers them to substrates, then promotes their degradation by the proteasome.6,40 There is also a study that speculates that the LIM (Lin11, Isl-1, and Mec-3) domain protein LIM domain only 7 (LMO7) may be the causative gene that causes AMD. The role of this gene is to mediate ubiquitination and proteasomal degradation and regulate the fibrotic response after injury.41,42
Role of autophagy in AMD
Autophagy is an important intracellular process for the degradation and removal of abnormal proteins and damaged organelles. It maintains the stability of the intracellular environment by forming autophagosomes to wrap and degrade intracellular waste, damaged proteins, and organelles.22 Generally, autophagy can be divided into three types: (1) Macroautophagy: A C-shaped double-layer membrane structure is generated in the cytoplasm, and the two ends extend to wrap part of the cytoplasm and organelles to form autophagosomes. Autophagosomes then combine with lysosomes to form autolysosomes. (2) Microautophagy: The lysosomal membrane is directly invaginated to wrap the components in the cytoplasm, and then form vesicles. (3) Chaperone-mediated autophagy: After being recognized by molecular chaperones, cytoplasmic proteins with special motifs combine with the lysosome-associated membrane protein type 2A on the lysosome membrane, and then enter the lysosome to be degraded.43,44 The autophagy process includes three key steps, the formation, fusion, and degradation of autophagosomes. When autophagosomes are formed, cells wrap waste or damaged materials into autophagic vesicles, forming a double-membrane structure. The class-1 phosphoinositide 3-kinase (PI3K) and its product phosphatidylinositol 3-phosphate are involved in this process.45–47 Together with protein kinase B (Akt) and the mammalian target of rapamycin (mTOR), it forms the PI3K/AKT/mTOR signaling pathway that is important for cell survival and growth.48–50 Rapamycin is a drug that inhibits the activity of mTOR in this step, thereby promoting autophagy.51 Subsequently, isolation membrane wraps around degradation vesicles to form autophagosome, and autophagosome fuses with lysosome membranes to form autolysosome, in which waste products and proteins are degraded into small molecules. Finally, the degradation products are released into the cytoplasm by transporters on the lysosomal membrane for reuse by the cell.52 Abnormal autophagy can lead to the occurrence and development of AMD, and it is currently found that it mainly affects the function of pigment epithelial cells, increases cell apoptosis, and causes damage to mitochondrial function. However, the specific mechanisms and interactions still require further studies to be fully understood.
impaired function in retinal pigment epithelial cells
The development of AMD is closely related to the abnormal function of retinal pigment epithelial cells. Abnormal autophagy may lead to the accumulation of harmful metabolites such as peroxides, lipid, and protein aggregates in pigment epithelial cells. These accumulated substances may negatively affect the normal function of the cells, leading to impaired function of the pigment epithelium, which contributes to the development of AMD.13,53,54
Defective autophagy
Abnormal autophagy can lead to increased apoptosis (programmed cell death). Normally, autophagy removes damaged or aged organelles and proteins, thereby maintaining cellular homeostasis. However when autophagy is impaired, cells cannot effectively remove harmful substances, which can lead to the accumulation of intracellular stress and damage, eventually triggering apoptosis.55 Impaired autophagy leads to the accumulation of drusen and lipids. The peroxisome proliferator-activated receptor-γ coactivator-1 alpha regulates autophagy and mitophagy.56 Studies have found that knocking out this gene in mice inhibits autophagy in the RPE and retina, leading to the accumulation of drusen-like substances.57 In addition, the FIP200 protein is involved in the formation of autophagosomes, and conditional knockout of this gene in mice leads to reduced autophagy, and these mice exhibit increased lipid accumulation with age.58,59
Mitochondrial function damage
Mitochondria are energy production centers within cells and important regulators of the autophagy process. Abnormal autophagy can lead to impairment of mitochondrial function, which in turn affects cellular energy metabolism and oxidative stress. Damage to mitochondrial DNA is associated with the development of AMD, and damage to mitochondrial DNA can lead to abnormal mitochondrial function, further aggravating cellular metabolic disorders and cell death.10,60
Genetic factors
Deficiency of calcium and integrin-binding protein 2 (CIB2) in mice has been found to lead to age-related retinopathy, including sub-RPE deposition, significant accumulation of drusen, and impaired visual function. CIB2 mutant mice have reduced lysosomal capacity and autophagic clearance, as well as increased expression of mTORC1, a negative regulator of autophagy. This defect was also observed in the RPE/choroid of dry AMD patients.61
Interaction and reciprocal regulation of UPS and autophagy
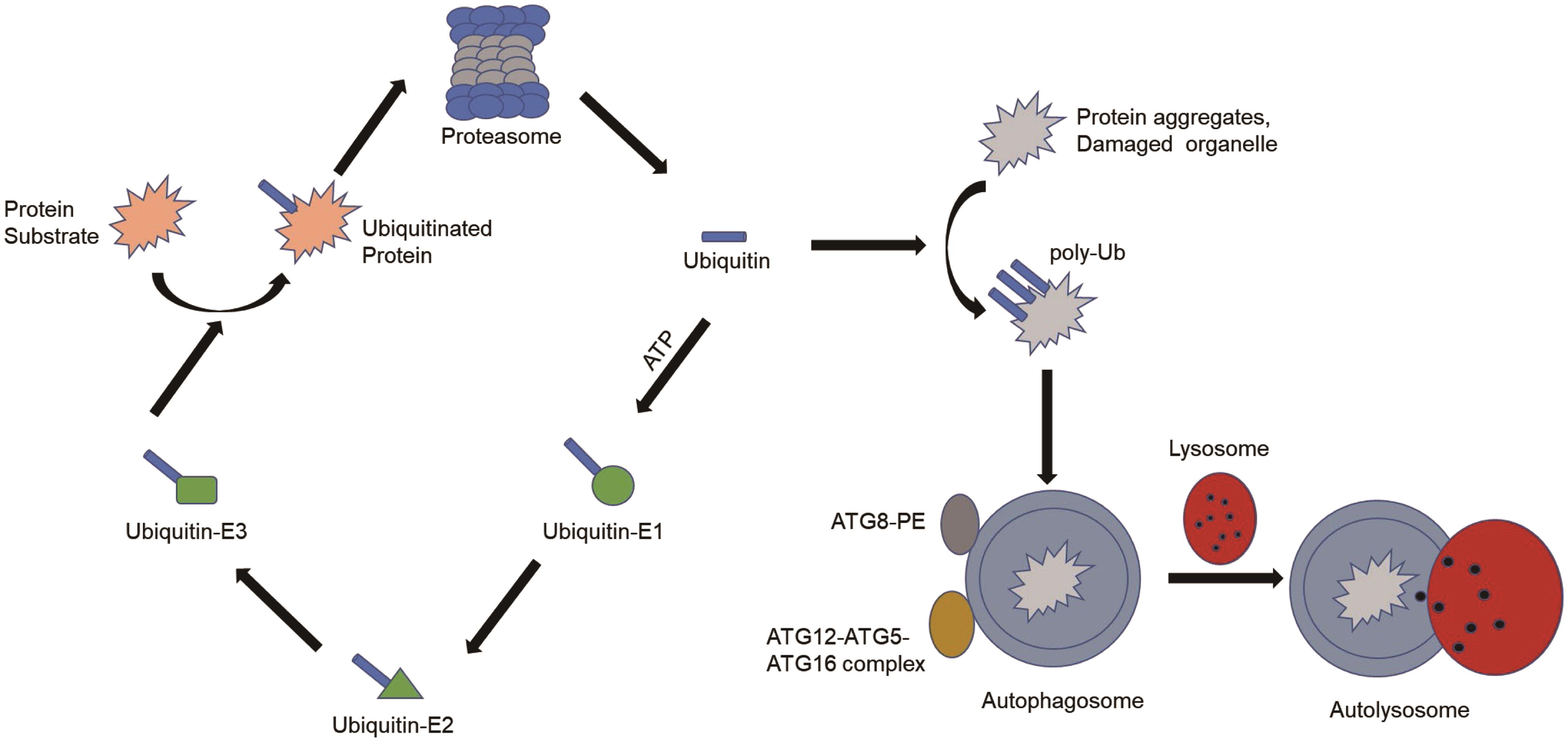
In the past, UPS and autophagy have been considered as two independent protein degradation mechanisms. Autophagy is a vesicular trafficking pathway specialized for the delivery of long-lived proteins, soluble or insoluble protein aggregates, and damaged organelles to lysosomes.62 The UPS, on the other hand, delivers soluble, short-lived, misfolded, or damaged proteins to the proteasome.63,64 Some recent studies have found that the two systems cooperate and influence each other. First, several molecules are shared by the UPS and autophagy (Fig. 1). Both systems use ubiquitin as a signaling molecule to tag regulators or substrates of proteins for degradation.65 Additionally, two ubiquitin-like binding systems are involved in the fusion process of autophagy. One is the ATG12/ATG5 system. The autophagy factor ATG12-ATG5 conjugate has E3 ligase-like activity and is required for lipidation by LC3 in autophagy.66,67 Another system includes ATG8 and its target molecules phosphatidylethanolamine and ATG4.68,69
ATG, autophagy-related genes; ATP, adenosine triphosphate; PE, phosphatidylethanolamine.
Inhibition of the UPS leads to a compensatory increase in autophagy through multiple mechanisms. For example, proteasome inhibitor MG132 and chemotherapeutic drug bortezomib inhibit proteasome activity, leading to increased expression of autophagy genes ATG5 and ATG7 and induction of autophagy.70,71 A cellular model of proteasome substrate accumulation due to combined knockdown of PSMD4/S5a and ADRM1 (two proteasome ubiquitin receptors) was also developed. This model reveals a compensatory autophagy pathway mediated by SQSTM1/p62-dependent clearance of accumulated polyubiquitinated proteins.72 In a study of RPE cells, treatment with low-level proteasome inhibitors activated autophagy by inhibiting the PI3K-Akt-mTOR pathway.73
Impaired autophagy also leads to the activation of the. UPS. Chemical inhibition of autophagy and small RNA-mediated knockdown of the ATG gene lead to upregulation of proteasome subunit levels and increased UPS activity in colon cancer cells.74 On the other hand, inhibiting autophagy can also impair UPS function. It has been found that after inhibition of autophagy, accumulated p62 inhibits the proteasome degradation of ubiquitinated proteins by delaying the delivery of ubiquitinated proteins to the proteasome.75
Conclusions and future perspectives
The UPS and autophagy have important roles in the pathogenesis of AMD. Their abnormal function or failure of interactions leads to the accumulation of abnormal proteins and damaged organelles, triggering inflammatory response and cell death, accelerating the progression of AMD. Although some research progress has been made on the role of ubiquitin-proteasome and autophagy in AMD, there are still many questions to be further studied and resolved. Here are some possible future research directions.
In-depth understanding of the interaction mechanism between ubiquitin-proteasome and autophagy
Further research on the interaction mechanism between ubiquitin-proteasome and autophagy, including key molecules and signaling pathways regulating autophagy and the ubiquitination process, will help reveal the specific role of ubiquitin-proteasome and autophagy in AMD and adjustment mechanism.
Development of therapeutic strategies targeting ubiquitin-proteasome and autophagy
Based on the in-depth understanding of ubiquitin-proteasome and autophagy, develop therapeutic strategies targeting these pathways, such as drug intervention, gene therapy, etc. This helps to restore the normal function of ubiquitin-proteasome and autophagy, reducing the accumulation of abnormal proteins and damaged organelles, thereby delaying the progression of AMD. Antiangiogenesis therapies targeting vascular endothelial growth factor (VEGF) have revolutionized the treatment of neovascular ocular diseases, including nAMD. This therapeutic strategy slows disease progression by inhibiting the formation and leakage of abnormal blood vessels.76 Treatment of macular neovascularization with VEGF-inhibiting biologics is a milestone in AMD treatment. However, response to treatment varies, and not all patients achieve or maintain consistently good vision over the long term. The pathophysiological mechanisms underlying this differential response are not fully understood. Furthermore, existing anti-VEGF strategies do not prevent the development of atrophy, which also leads to vision loss in the long term. AMD represents a spectrum of diseases comprising distinct phenotypes with distinct pathogenesis. Therefore, tailoring treatments to specific phenotypes and stages may be the key to preventing irreversible vision loss in the future.77,78
Based on the important roles of ubiquitin-proteasome and autophagy in AMD, modulating ubiquitin-proteasome and autophagy functions could also be a potential therapeutic strategy. One strategy is to promote the degradation of abnormal proteins and damaged organelles by activating the autophagy pathway. This can be achieved through drug intervention, nutritional regulation, or gene therapy. Some drugs have been found to modulate the activity of the autophagy pathway, such as autophagy inducers such as rapamycin and chloroquine.61,79 These drugs promote the degradation of abnormal proteins and damaged organelles, reducing inflammation and cell death.80,81
Another strategy is to promote the degradation of target proteins by modulating ubiquitin-proteasome function. This can be achieved through methods such as drug intervention or gene therapy. Several drugs have been found to modulate ubiquitin-proteasome function, such as E3 ligase inhibitors and ubiquitin protease activators. For example, the small-molecule compounds 33–11 and KH-4-43, proteolysis targeting chimeras, and some patented compounds, such as panepophenanthrin, himeic acid A, and dimeric sterols (manadosterols A and B), etc.82,83 These drugs can enhance the degradation of ubiquitinated proteins and reduce the accumulation of abnormal proteins. However, further studies are needed to evaluate the efficacy and safety of these drugs in the treatment of AMD.
Explore the relationship between ubiquitin-proteasome and autophagy and other diseases
In addition to AMD, ubiquitin proteasomes and autophagy also have important roles in other ocular diseases and neurodegenerative diseases. Further research on the association between UPS and autophagy and these diseases will help reveal their common mechanisms and therapeutic strategies in different diseases.
Development of new assays and biomarkers for UPS and autophagy
Development of sensitive and specific assays and biomarkers for the assessment of ubiquitin-proteasome and autophagy function and activity. This helps in the early diagnosis and monitoring of the development of AMD and provides a basis for the selection of treatment strategies. In general, future research should continue to deeply explore the mechanism of UPS and autophagy systems in AMD, and translate the results of laboratory research into clinical applications. Through in-depth research and the development of innovative treatment strategies, we can provide AMD patients with better treatment options, reduce their suffering, and improve their quality of life.
Abbreviations
- Akt:
protein kinase B
- AMD:
age-related macular degeneration
- ATG:
autophagy-related genes
- ATP:
adenosine triphosphate
- CIB2:
calcium and integrin-binding protein 2
- E3 ligase:
ubiquitin ligase
- mTOR:
mammalian target of rapamycin
- nAMD:
neovascular AMD
- PE:
phosphatidylethanolamine
- PI3K:
phosphoinositide 3-kinase
- RPE:
retinal pigment epithelium
- UBE3D:
ubiquitin protein ligase E3D
- UPS:
ubiquitin-proteasome system
- VEGF:
vascular endothelial growth factor
Declarations
Acknowledgement
We are grateful to all the participants and their families for their generosity and support.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) fund (81670870), the Beijing-Tianjin-Hebei Special Project (J200014), and the Science and Technology Innovation Project of Chinese Academy of medical sciences (2019-RC-HL-019) (to LH); and National Key RD Program of China (2020YFC2008200) (to MZ). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The manuscript was submitted during Dr. Mingwei Zhao's term as the Editor-in-Chief of Nature Cell and Science. The authors have no other conflict of interest to declare related to this publication.
Authors’ contributions
Contributed to study concept and design (LzH and MwZ), acquisition of the data (NdX,JrL and XdC), drafting of the manuscript (NdX and JrL), critical revision of the manuscript (LzH and MwZ), supervision (LzH).

 Author information
Author information