Introduction
Neurons can receive external and/or internal stimuli as well as conduct nerve impulses. In addition, specific neuroinjuries can interrupt the critical connections. Some glial cells, such as Schwann cells and/or oligodendrocytes, can wrap around neural axons to form myelin structures, which can protect neurons and/or facilitate the conduction of impulses. Glial cells have potassium channels that respond to extracellular changes such as pH variations. Meanwhile, astrocytes are a diverse class of glial cells restricted to the central nervous system. Peripheral nerve injuries often arise from a consequence of inflammatory and/or traumatic reasons, resulting in heavy social burdens,1 which are unfortunately often associated with poor recovery.2 Prolonged periods of denervation may decline the capability for axon regeneration. Interestingly, it has been shown that the roles of the corneal axon-ensheathing Schwann cells may be critical in homeostatic corneal epithelial cell renewal.3 The mechanism may be relevant to the activation of the phosphoinositide 3-kinase (PI3K)/AKT/phosphatase and tensin homolog signaling pathway.4 The cornea is innervated by the ophthalmic branch of the trigeminal nerve, entering the cornea at the limbus and penetrating into the corneal epithelium, which is considered to be responsible for the activation of tearing and blink reflexes and repair. In general, the corneal sensory nerves protect the cornea from injury. When the protective corneal sensory innervation is lost after infection, trauma, and/or intracranial tumors, permanent blindness would occur via the repetitive microtraumas that induce opacification of the cornea, which is known as neurotrophic keratopathy and is increasing worldwide.5 Recombinant human nerve growth factor (NGF) can be applied for the treatment of this neurotrophic keratopathy; however, it is not effective in all patients.6
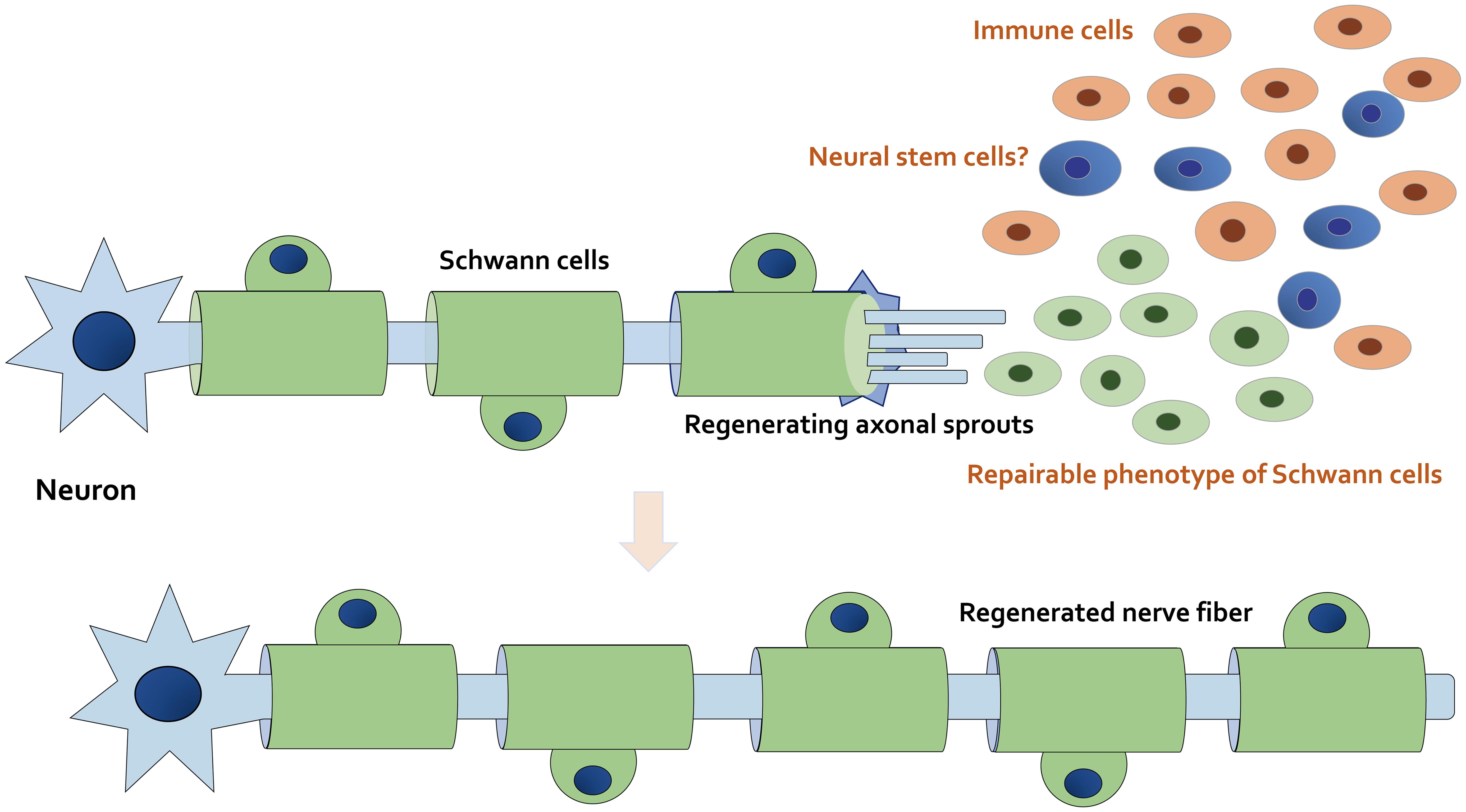
It has been reported that there are biological mechanisms capable of assisting natural retrieval within the nervous system.7 In particular, severe nerve injuries may require embedding material as a scaffold to guide neuroregeneration.8 Accordingly, it is important to explore better treatments capable of encouraging nerve regeneration to accept the repair of function. A series of neurotrophic growth-inducing factors, such as NGF, brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor, and vascular endothelial growth factor, are secreted by glial cells to encourage the survival of neurons.9 Additionally, Schwann cells are stimulated by an advanced myelinating condition to a repairable phenotype.10,11 In this situation, some Schwann cells may fail to come into contact with the adjacent axons, thus resulting in fundamental alterations of the neuron signaling environment. This exceptional feature of Schwann cells can provide a forceful regeneration ability within nerves (Fig. 1).
The axon and myelin sheath in the distal stump may degenerate after nerve injury. The mature myelinating Schwann cells dedifferentiate into immature Schwann cells and proliferate. The fragmentation of axons and myelin that occurs at the injury site may induce the dedifferentiation of Schwann cells. Immune cells including macrophages migrate to the site of the lesion, and Schwann cells with the proliferating repairable phenotype can remove myelin debris. After the debris has been removed, dedifferentiated Schwann cells contribute to guide axonal sprouting to support axon regeneration. Note that some critical pathways have been omitted for clarity.
Once engaged as scaffolds for regenerative treatment, natural biomaterials might be better than artificial polymers, which have been utilized to make intricate supports such as several organ scaffolds. Bionic nerve scaffolds are able to provide a suitable microenvironment for nerves. As structural reconstruction may play a significant role in nerve repair,12,13 certain signaling pathways can influence the recruitment, adhesion, and migration of glial cells based on the situation of nerve tissues.14 Consequently, extracellular matrix-based nerve regenerative medicines for repairing nerve injury and/or neuroprotection have been increasingly studied.15 In particular, bioscaffolds can be used to keep the best bioelasticity and mechanical properties to improve the adhesion, differentiation, and proliferation of neuronal cells in addition to the enhanced repair of nerve injuries.16 However, existing neuronal biomaterials are incompletely effective in treating peripheral nerve injuries.17 Although effective repair of the nervous system has been broadly explored, it is still imperative to develop better-quality bioscaffolds.
PI3K/AKT signaling pathway in Schwann cells is associated with autophagy and neural regeneration
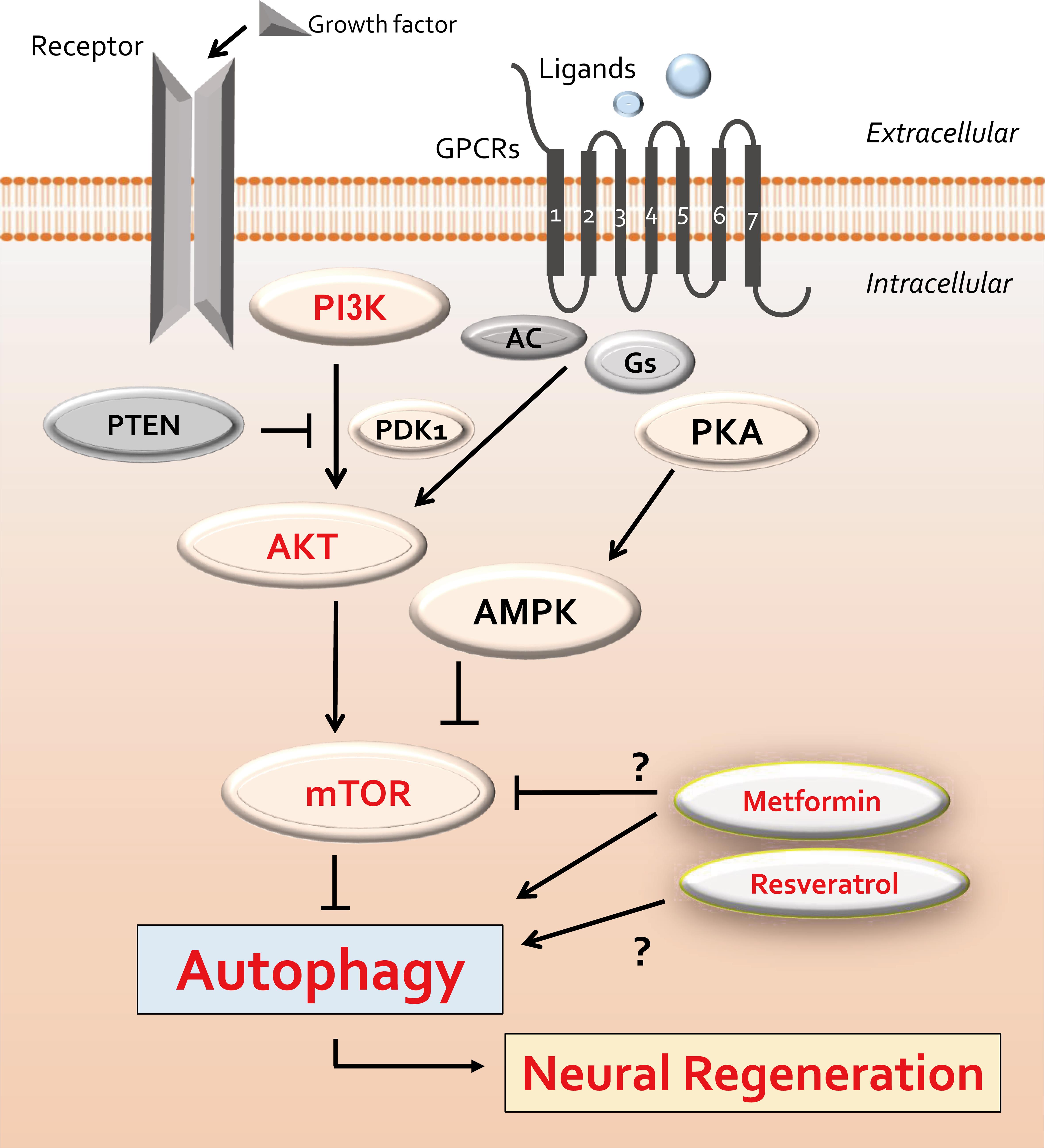
Autophagy is a significant process that breaks down proteins, lipids, and organelles, and it also plays an important role in maintaining homeostasis via adjusting molecular activities in response to various stresses.18 A growing number of investigations have recognized the key role of autophagy in controlling cell migration, with varied functions in different cell types.19 Under stress conditions, autophagy may play either a cytotoxic or a cytoprotective role in cellular homeostasis and survival.20 In the context of neuroregeneration, autophagy also may be crucial in the remodeling of synaptic elements to maintain neuronal function.20 The autophagy process, including initiation, progression, and termination, is modulated by the mammalian/mechanistic target of the rapamycin (mTOR) signaling pathway.21 The mTOR signaling pathway is also involved in regulating cell growth, proliferation, and life span.22 In addition, enhancing autophagy exhibits a crucial role in regulating the migration of Schwann cells after several nerve injuries.23 For example, it has been reported that attenuation of neuronal apoptosis in traumatic brain injury can be achieved by supporting autophagy through the PI3K/AKT pathway,24 which is an upstream pathway of mTOR signaling. In general, the PI3K/AKT/mTOR signaling pathway has been shown to be involved in the control of autophagy (Fig. 2).25 Therefore, the PI3K/AKT/mTOR pathway plays important roles in controlling cell differentiation, proliferation, survival, metabolism, and autophagy of stem cells.26 Moreover, the PI3K/AKT/mTOR signaling pathway may also play an indispensable role in preserving cell stability by declining the expression of apoptosis-related molecules such as caspase-3 in neurons. Autophagy seems to be predominantly repressed via the PI3K/AKT/mTOR signaling pathway.27 In contrast, metformin has been demonstrated to increase the level of autophagy and to prevent the migration of several precursor cells via activation of the PI3K/AKT/mTOR pathway (Fig. 2).28
A few example compounds known to act on autophagy are also shown. The arrowheads indicate stimulation, whereas hammerheads show inhibition. Note that several important molecules have been omitted for simplicity. AC, adenylyl cyclase; PDK1, phosphoinositide-dependent kinase-1; PI3K, phosphoinositide-3 kinase; PKA, protein kinase A; AMPK, adenosine monophosphate-activated protein kinase; mTOR, mammalian/mechanistic target of rapamycin; PTEN, phosphatase and tensin homolog deleted on chromosome 10.
Recent investigations have shown that autophagy comprises the phagocytosis of myelin, suggesting that disorders of autophagy may impair myelin clearance.29 Interestingly, resveratrol promotes recovery from sciatic nerve crush injury by accelerating the myelin clearance process by promoting autophagy of Schwann cells.30 Peripheral nerve regeneration may be thoroughly related to the removal of damaged myelin by Schwann cells, which results in Schwann cell-mediated autophagy as a neuroprotective role for repairing damaged neurons.29 The effective removal of injured myelin sheaths is indispensable for certifying remodeling or the functional recovery of neurons succeeding several peripheral nerve injuries. Accordingly, a deficiency of Schwann cell autophagic activity might result in the significant scar formation of nerves. Furthermore, modulating autophagy can be a powerful approach for improving nerve function from the outcomes of neuroinjury.31 As mentioned above, Schwann cells can promote the survival of damaged neurons and/or axon regeneration by activating the PI3K/AKT pathway, which may also offer effective strategies for the treatment of ischemic stroke.32 The coculture of Schwann cells and endothelial cells can stimulate the release of NGF and vascular endothelial growth factor for the development of nerve grafts in nerve regeneration via the PI3K/AKT pathway.33 Additionally, the specific PI3K/AKT signaling pathway in Schwann cells may be necessary for the development of neuromuscular junctions.34 Moreover, regulation of the PI3K/AKT signaling pathway may serve as a potential therapeutic target for the treatment of diabetic peripheral neuropathy.35,36
Certain bioscaffolds can support the roles of Schwann cells
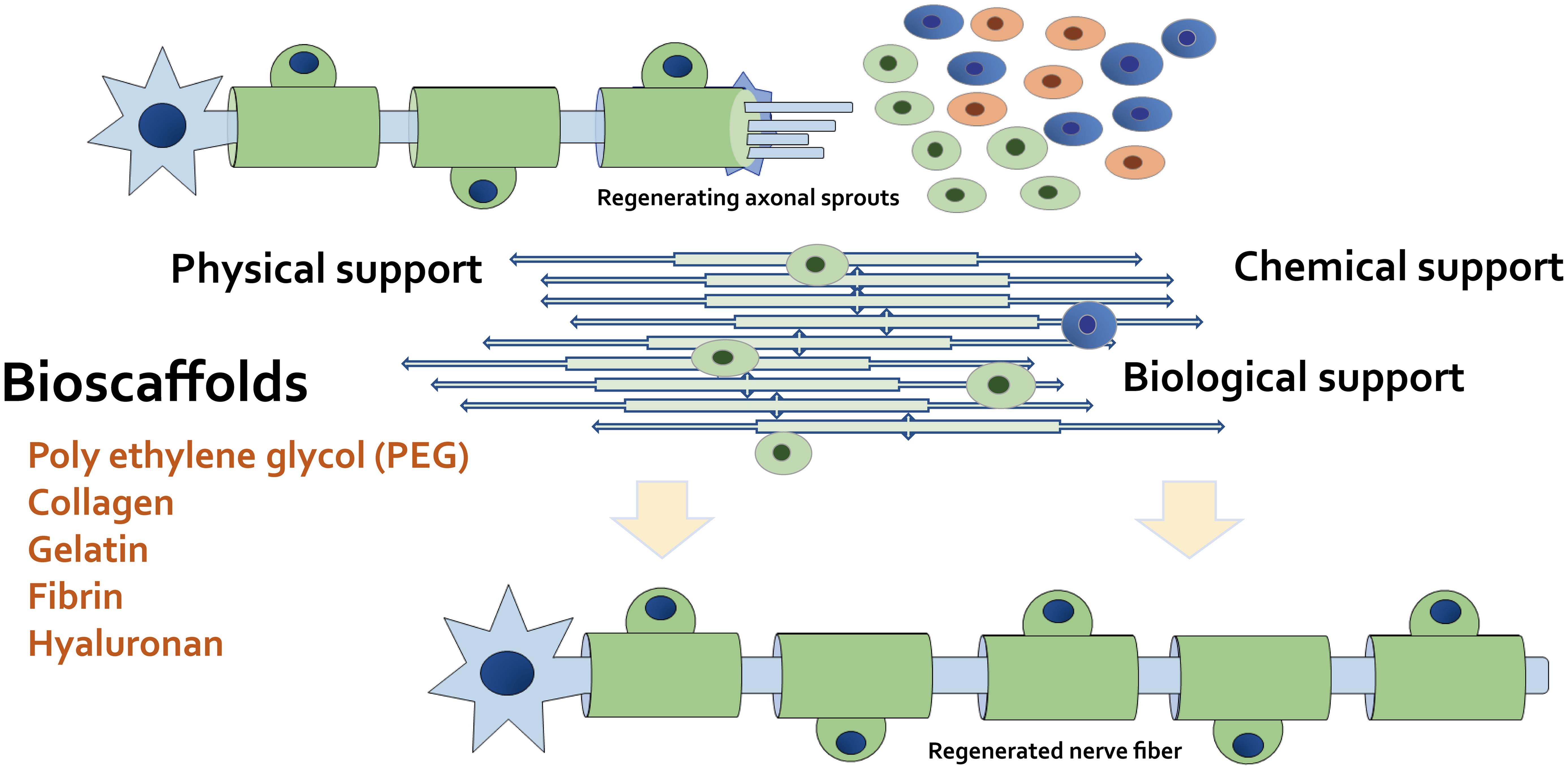
After nerve injury, the injured axons can become distorted and the myelin sheath can crumble.37 In some features of nerve regeneration, Schwann cells can play a fundamental part in the nerve regeneration process. Simultaneously, the stimulated Schwann cells can initiate to elongate and arrange in a line within the residual endoneurial tube to create specific bands, which can provide a pathway for the growth direction of regenerating axons.38,39 Following axonal regeneration, Schwann cells can wrap around the new axons to renovate myelin sheaths. The stimulated Schwann cells can participate in the removal of cellular debris, which can assist myelination through the achievement of autophagy.40 Some myelinating Schwann cells also can be employed to support myelin repair after nerve injury.41 In the central nervous system, oligodendrocyte precursor cells can differentiate into remyelinating Schwann cells in response to nerve injury and/or demyelination.42 Therefore, neuron regenerative medicine should aim to advance the potential of spontaneous remyelination for the most wanted nervous system.43 Without myelin sheath repair, further neurodegeneration will take place. It is likely that primary remyelination would spontaneously occur upon nerve injury in healthy individuals. However, a biological intervention that encourages remyelination can have an indispensable impact on a patient’s life by inhibiting extra neurodegeneration. Once nerve injury occurs, Schwann cells are critically involved in several stages of the following regenerative processes. A noteworthy challenge in this field may be how to reconstruct the microenvironment of the Schwann-cell/oligodendrocyte/axon interaction. Recent progress in this research field related to the elucidation of the intrinsic mechanism of nerve repair has augmented the focus on the design of scaffold materials that can promote the microenvironment and/or the repair processes.44 Consequently, some engineered scaffolds with neuronal grafts/cells have been reported to successfully work as an ideal scaffold to promote axon regeneration after segmental nerve defects.45 For example, the ability of scaffolds to sustain neuronal health at an appropriate time point has been shown to maintain the regenerative capacity to enhance the extent of recovery.45 Some scaffolds also can provide continuous regeneration-related neurotrophic factors that support regenerating axons to assist their functional recovery (Fig. 3).
These bioscaffolds have potential as physical, chemical, and biological supports to repair cells. Certain bioscaffolds can additionally promote the remyelination of Schwann cells, which can be used as a conductive platform for neural tissue engineering. Example materials suitable for a bioscaffold also are shown. Note that several important activities such as inflammatory reactions have been omitted for simplicity.
In contrast to axons in the central nervous system, damaged peripheral nerves have demonstrated the capacity to regenerate neurons mainly due to the helpful population of Schwann cells, which are the major glial cells in the peripheral nervous system. Schwann cells have a unique ability to myelinate axons, which can also promote nerve regeneration by discharging several neurotrophic factors.46 In addition, Schwann cells play a significant role in peripheral nerve restoration not only in the clearance of myelin sheath debris but also in the remyelination of axons and/or their adhesion.47,48 The basement membrane constructed by Schwann cells enables the attachment of axons and Schwann cells via the extracellular matrix proteins such as fibronectin and/or laminin, encouraging further neurite outgrowth.49 On the other hand, myelinating Schwann cells also can provide several neurotrophic factors such as brain-derived neurotrophic factor and/or NGF in addition to the receptors of these factors, which are indispensable for the elongation of axonal growth after nerve injury. In other words, matrix proteins can provide an adhesive element for axons to Schwann cells, while neurotrophic factors can enhance nerve regeneration.50 Accordingly, establishing a certain microenvironment through the effect of neurite outgrowth-promoting factors is essential for nerve regeneration. Nerve regeneration fails without either appropriate neurite outgrowth and/or neurotrophic factors, which also suggests that a crucial relationship between growth-promoting matrix and neurotrophins is required for complete nerve regeneration. Schwann cells play an important role in driving axon elongation by forming aligned tubular guidance structures, which promptly arise in distal nerve segments.51 However, Schwann cells may frequently fail to interlope large-gap scaffolds, thus resulting in poor outcomes. Therefore, the development of additional connecting strategies is needed to induce the full neuroregenerative ability within Schwann cells.51
The concept of bioscaffolds for superior neuroregeneration with mesenchymal stem cells or Schwann cells
Although therapeutic efficacy is compromised by inefficient cell delivery, the transplantation of mesenchymal stem cells and/or Schwann cells has been shown to be a favorable therapy using regenerative medicine for several nerve injuries. In this procedure, the mechanical processability of various synthetic polymers such as polyethylene glycol (PEG) might play an essential role in scaffold preparation for the transplantation, which should be investigated for different constructions such as aligned microfibers to regulate the activities of mesenchymal stem cells and/or Schwann cells. An ideal material should match the properties of the target nerve circumstances. In the pursuit of an effective nerve tube, various physical and biological strategies have been employed.52 For superior neuroregeneration, specific bioscaffolds with the capability of physical, chemical, and/or biological support to neuronal cells including stem cells and/or Schwan cells are indispensable (Fig. 3).
Collagen scaffolds have increased the retention of mesenchymal stem cells in the lesion site. In addition, more mesenchymal stem cells have been identified in the site when transplanted with collagen scaffolds, resulting in superior neural functional recovery. It also has been suggested that collagen scaffolds can support the survival of grafted stem cells and/or Schwann cells.53 The collagen provides local physical retention, mimics the extracellular scaffold, provides physical cues for cell spreading, and supports the survival of grafted stem cells,53 thus providing an appropriate microenvironment to maintain cell attachment and/or cell proliferation. In fact, collagen scaffolds support the survival and differentiation of grafted cells, suggesting that the combination of mesenchymal stem cells and collagen scaffolds might be a prominent therapeutic treatment for brain injury.53 Moreover, collagen may provide a favorable environment for nerve cells. For example, differentiation of PC12 cells and mouse neural stem cells has shown decent elongation and/or alignment of neurite outgrowth in collagen.54 Additionally, the PI3K/AKT pathway has been demonstrated to be a signaling mediator of collagen in Schwann cells.55
Gelatin methacrylate-based hydrogels are also gaining much attention as possibly good implantable tools for tissue manufacturing purposes owing to their advantageous biofunctionality. For example, a gelatin-based hydrogel system for the bioactive delivery of vascular endothelial growth factor has been established by assessing AKT phosphorylation in Schwann cells.56 However, applications employing gelatin methacrylate hydrogels are presently limited by their low mechanical strength.57 A new tactic based on gelatin scaffolds for tissue transplantation in order to repair neural tissue injuries should be examined and developed. Accordingly, the gelatin methacrylate-based hydrogel-cell combinations might play a significant role in repairing neural tissue injuries, which could speed up the improvement of clinically relevant applications.57 In addition, combination therapy using polycaprolactone/gelatin scaffolds has the potential to repair the injured spinal cord as well as to reduce secondary damage.58 For example, differentiation of endometrial stem cells on the scaffolds into motor neuron-like cells is of great value for the regeneration of injured spinal cords,58 where Schwann cells secrete several neurotrophic factors that are indispensable for neural differentiation.
An important transplantation vehicle, fibrin, can improve the differentiation of mesenchymal stem cells into Schwann-like cells and deserves further research. In fact, fibrin has shown great promise as a cell transplantation vehicle for the treatment of some types of nervous system injuries.59 The fibrin hydrogel can play a protective role throughout the cell transfer process by providing cell attachment sites and/or signals, which has been demonstrated as a suitable strategy for the preparation of neural progenitor cells with favorable outcomes.60 The continuous use of a non-neurotoxic fibrin matrix also has been shown to be a convenient strategy for a better transplantation outcome in cell delivery.60 In addition, an aligned fibrin hydrogel has been revealed to upregulate the expression of regeneration-associated genes, which also activate the PI3K/AKT signaling pathways in regenerated nerve cells.61 PI3K/AKT signaling guarantees neuronal survival and axonal growth mediated by several neurotrophic factors, which are stimulated during the process of axonal regeneration.61
Hyaluronan-based hydrogels are among the most promising neural tissue engineering materials because of their biocompatibility and the immunomodulation capabilities of their degradation byproducts.62 For instance, hyaluronic acid is an abundant extracellular matrix component in soft tissues throughout the body and has found wide adoption in tissue engineering.63 Moreover, multimodular microfilaments with hyaluronic acid are effective in promoting directed axon growth and regeneration in the nervous system.64 Furthermore, an injectable and bioresorbable hydrogel blend of hyaluronan and methylcellulose can improve the distribution, viability, and functional repair of neural stem and progenitor cells.65 Additionally, high-molecular-weight hyaluronan can transduce the signal for the PI3K/AKT pathway.66 It also has been shown that hyaluronan is an excellent cell scaffold to improve the treatment efficiency of mesenchymal stem cells, and the therapeutic mechanism is through activation of the PI3K/AKT pathway.67
PEG-based hydrogels are used widely in exploratory tissue engineering applications. PEG and hyaluronan derivative-crosslinked hydrogels are nontoxic towards primary Schwann cells and are tunable to soft tissues such as those found in the central and/or peripheral nervous system.68 Additive manufacturing to the crosslinked hydrogels offers scaffold morphologies biocompatible to that of primary Schwann cells.69 In addition to a significant increase in functional recovery in mice, axon elongation and/or remyelination have been observed with the use of a PEG tube for a cell delivery platform.70 In addition, PEG-based hydrogels can be engineered for the alignment of nerve cells in a three-dimensional manner, which results in linear neurite extension for the treatment of acute spinal cord injuries.71 Moreover, multiarm thiolated PEG hydrogels can effectively improve the proliferation of mesenchymal stem cells via the PI3K/AKT/glycogen synthase kinase-3 beta signaling pathway.72
Perspectives
It has been shown that blocking the PI3K/AKT/mTOR pathway worsens neuronal damage in the primary culture of cortical neurons.73 Consistently, mesenchymal stem cell transplantation successfully repaired the function of motor neurons after spinal cord injury by activating the PI3K/AKT/mTOR pathway.73 Also, exosome-loaded electroconductive hydrogels have been shown to synergistically enhance oligodendrocyte differentiation of neural stem cells and increase axon outgrowth via the PI3K/AKT/mTOR signaling pathway.74 Moreover, to cure spinal cord injury, it is critical to achieve an anti-inflammatory microenvironment through the PI3K/AKT/mTOR signaling pathway.75 Therefore, by reducing early inflammation, myelin-associated axonal regeneration can be promoted to recovery after nerve injury. In particular, certain scaffolds have been shown to enhance neuronal and/or oligodendrocyte differentiation of neuronal stem cells, which promote the elongation of axons via modulation of the PI3K/AKT/mTOR pathway. Consequently, PI3K/AKT/mTOR signaling has been demonstrated to encourage the transplantation of mesenchymal stem cells through neuroprotective and/or immunomodulatory activity.76 The combination of mesenchymal stem cells and Schwann cells appears to represent a promising therapeutic vision. In particular, the interaction between stem cells and supporting cells appears to be crucial for neural regeneration.77 However, the differentiation of mesenchymal stem cells into neuronal and/or glial cells still remains an issue of discussion. Interestingly, mesenchymal stem cells derived from different tissues including the umbilical cord, bone marrow, and/or adipose tissue can be induced into Schwann-like cells under special conditions.78 In addition, the possibility of creating neural guidance conduits through connecting collagen hydrogel to Schwann-like cells with significant therapeutic potentials has been demonstrated to facilitate the functional recovery and axonal regeneration of rat facial nerves.79 An advantage of the use of mesenchymal stem cells is their ease of isolation from multiple sources.
As for corneal injury, it has been shown that sodium hyaluronate in eye drops may provide a promising treatment for superficial corneal abrasion caused by mechanical damage.80 This makes sense because hyaluronate can support bioscaffolds for better neuroregeneration, as shown in Figure 3. Likewise, metformin and/or resveratrol can promote the recovery of corneal injuries because they can improve autophagy (Fig. 2). In fact, the efficacy of metformin eye drops against the alkali-induced corneal neovascularization model has been reported.81 Furthermore, metformin can also protect against retinal injury through mitochondrial fusion and reduced reactive oxygen species generation.82 Resveratrol also has been shown to be protective against ischemia-reperfusion injury in the murine eye/retina.83 The process of neuroregeneration is rather complicated due to the intricate communication between neuronal cells and glial cells. Widespread strategies that target different pathological processes might produce a better response with respect to neural regeneration. Future research should focus on the roles of the PI3K/AKT signaling pathway in different glial cells under physiological and/or pathological conditions. All efforts would support the development of novel therapeutic strategies. In particular, understanding of the molecular mechanisms that can direct the process of myelin sheath formation might also be important to speed up the development of therapeutics with certain bioscaffolds for neural regeneration.
Abbreviations
- AC:
adenylyl cyclase
- PDK1:
phosphoinositide-dependent kinase-1
- mTOR:
mammalian/mechanistic target of rapamycin
- NGF:
nerve growth factor
- PEG:
polyethylene glycol
- PI3K:
phosphoinositide 3-kinase
Declarations
Acknowledgement
None.
Funding
None.
Conflict of interest
The authors have no conflicts of interest related to this publication.
Authors’ contributions
Study concept (NS, SM), and drafting and editing of the manuscript (NS, KT, SY, HS, YI, SM). All authors revised the manuscript critically and approved the version to be published.

 Author information
Author information