Introduction
Chimeric antigen receptor (CAR)-regulatory T cells (Tregs), which combine the intrinsic stability of Tregs with the engineered antigen specificity of CAR technology, provide a rational and innovative strategy to induce long-lasting immune tolerance. CAR-Tregs represent a promising frontier in cell-based immunotherapy, offering precise and durable immunosuppression for non-malignant immune-mediated conditions such as transplant rejection, autoimmune diseases, and chronic inflammation.1 Unlike CAR T cells, which are designed to eliminate malignant cells, CAR-Tregs employ targeted immune modulation through antigen-specific suppression, thereby circumventing the systemic immunosuppression and adverse effects commonly associated with conventional therapies.2,3 CAR-Tregs are produced by isolating autologous Tregs, genetically engineering them to express CARs, and reintroducing them into the patient. These CARs enable major histocompatibility complex (MHC)-independent recognition of specific surface antigens, such as proteins, carbohydrates, or glycolipids, facilitating targeted immunosuppression.4,5 Upon antigen engagement, CAR-Tregs suppress immune responses through multiple mechanisms, including the secretion of interleukin (IL)-10, transforming growth factor-beta (TGF-β), and IL-35, and through interaction with dendritic cells to inhibit immune activation. Like their effector CAR T cell counterparts, CAR-Tregs demonstrate hallmark features of living therapeutics, including antigen-driven expansion, tissue homing, persistence, and dynamic responsiveness to immune challenges.6,7 Preclinical studies have highlighted the therapeutic potential of CAR-Tregs in diverse disease models, including allograft rejection, graft-versus-host disease (GvHD), and autoimmune conditions such as type 1 diabetes (T1D) and multiple sclerosis (MS). In transplantation models, human leukocyte antigen (HLA)-specific CAR-Tregs have extended graft survival by promoting antigen-specific tolerance. In T1D models, GAD65-targeted CAR-Tregs effectively trafficked to pancreatic islets and maintained immunoregulatory function for extended periods post-infusion.8 Although they failed to prevent disease onset in non-obese diabetic/Ltj mice, their persistence in secondary lymphoid organs reflected robust survival and immunomodulatory capacity. In vitro, insulin-specific CAR-Tregs retained a suppressive phenotype and inhibited effector T-cell proliferation, reinforcing their potential for antigen-directed immune regulation.9 Further validation comes from inflammatory bowel disease models, where TNP-specific CAR-Tregs conferred antigen-dependent and dose-responsive suppression of colitis, affirming their capacity for in vivo expansion and functional adaptability.10 Similarly, A2-CAR-Tregs ameliorated alloimmune-mediated skin injury, while AAV-CAR-Tregs maintained Treg phenotypes, dampened effector T-cell activity, and attenuated inflammation through sustained cytokine secretion and enhanced transgene expression.11 In addition to direct antigen recognition, CAR-Tregs exhibit bystander suppression, attenuating unrelated immune responses in the surrounding microenvironment. They further promote infectious tolerance by inducing a regulatory phenotype in neighboring immune cells, thereby amplifying and prolonging their immunosuppressive effects.12 Through these interconnected mechanisms, CAR-Tregs deliver precision immunomodulation. Nevertheless, one of the principal challenges in CAR-Treg therapy is maintaining phenotypic stability. Inflammatory milieus can downregulate forkhead box protein P3 (FOXP3) expression, predisposing Tregs to transdifferentiate into pro-inflammatory effector T cells.13 To address this, strategies such as FOXP3 overexpression and careful antigen selection are under investigation. Furthermore, CAR-Treg manufacturing is technically complex, requiring the isolation of highly pure Treg populations and preservation of their functional identity following genetic modification.6 Despite these hurdles, CAR-Tregs offer considerable translational promise. Their capacity to induce targeted, long-lasting immune tolerance while preserving systemic immune competence represents a major advancement over existing immunosuppressive therapies. Ongoing innovations in CAR design, gene delivery systems, and safety controls continue to accelerate the clinical translation of CAR-Tregs for autoimmune diseases, transplantation tolerance, and gene therapy applications.14 Ultimately, CAR-Tregs signify a paradigm shift in the treatment of immune-mediated disorders, moving from generalized immunosuppression to personalized, antigen-specific immune modulation. As living therapeutics, they offer the potential to reshape immune tolerance strategies and revolutionize the management of complex immune-driven diseases.
This review aims to comprehensively examine the evolution, mechanisms, clinical progress, and future prospects of CAR-Treg therapy as a next-generation approach for restoring immune tolerance in autoimmune and inflammatory diseases. Beginning with the conceptual foundation derived from CAR-T cell therapy, we discussed how the success of CAR-T cell technology in oncology has inspired the engineering of Tregs for targeted immune suppression. The review further outlines the molecular design of CAR constructs, preclinical and clinical developments, mechanisms of action, and therapeutic applications across diseases such as rheumatoid arthritis, systemic lupus erythematosus (SLE), T1D, MS, and Crohn’s disease.
Engineering CAR-Tregs: Insights from CAR T cell platforms
The evolution of CAR T cell therapy has laid a critical foundation for the development of CAR-Tregs. While CAR T cells aim to kill cancer cells and CAR-Tregs aim to suppress immune responses, both use similar CAR design, manufacturing methods, and translational approaches.4,15
At the molecular level, both CAR T cells and CAR-Tregs utilize a modular receptor architecture consisting of an extracellular single-chain variable fragment (scFv) for antigen recognition, a hinge and transmembrane domain, and intracellular signaling components. The linker connecting the scFv to the activation domain is typically rich in glycine and serine residues to confer structural flexibility.16 While CD3ζ is a common activation motif across both platforms, the selection of co-stimulatory domains is tailored to the therapeutic intent. In CAR T cells, co-stimulatory signals such as CD28 or 4-1BB (CD137) are incorporated to enhance cytotoxicity, persistence, and proliferation. In contrast, CAR-Tregs predominantly favor CD28, as it reinforces FOXP3 expression and supports their regulatory phenotype and suppressive function.4
Since their inception, CAR T cell therapies have undergone iterative refinements through successive generations, each improving structural design, signaling fidelity, and therapeutic efficacy.4 As living therapeutics, CAR T cells exhibit high antigen specificity, prolonged persistence, and robust clinical responses. In patients with B-cell malignancies—including acute lymphoblastic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, and multiple myeloma—CAR T cells have been detected in peripheral circulation for three to six months post-infusion, demonstrating their capacity for long-term immune surveillance.17,18 These clinical successes culminated in the U.S. Food and Drug Administration approval of six CAR T cell therapies beginning in 2017, marking a watershed moment in cancer immunotherapy.6 Products approved by the U.S. Food and Drug Administration, such as Kymriah (tisagenlecleucel), Yescarta (axi-cel), Tecartus (brexu-cel), Breyanzi (liso-cel), and Abecma (ide-cel), primarily target CD19 or BCMA antigens and offer transformative, and in some cases curative, treatment options for patients with relapsed or refractory hematologic malignancies.19 Ongoing clinical trials continue to expand the therapeutic horizon of CAR T cells, while simultaneously informing the design, optimization, and clinical translation of CAR-Tregs in immune-mediated disorders (Table 1).20
FDA-approved CAR T cell-based therapies20
| Therapy | Target antigen | Target approved indication | Month and year of approval | Clinical trials | Adverse effects |
|---|---|---|---|---|---|
| Kymriah (Tisagenlecleucel) | CD19 | Pediatric/ young adult B-ALL; adult DLBCL | August, 2017 | NCT02435849 (ELIANA trial); NCT02445248 (JULIET trial) | CRS, Neurotoxicity, B-Cell aplasia |
| Yescarta (Axicabtagene ciloleucel) | CD19 | DLBCL, PMBCL, FL | October, 2017 | NCT02348216 (ZUMA-1); NCT03105336 (ZUMA-5) | CRS, Neurotoxicity |
| Tecartus (Brexucabtagene autoleucel) | CD19 | MCL, adult B-ALL | July, 2020 | NCT02601313 (ZUMA-2); NCT02614066 (ZUMA-3) | CRS, Neurotoxicity |
| Breyanzi (Lisocabtagene maraleucel ) | CD19 | DLBCL, FL, CLL/SLL | February, 2021 | NCT02631044 (TRANSCEND-NHL-001); NCT03331198 (TRANSCEND-CLL-004) | Lower rates of CRS and Neurotoxicity |
| Carvykti (Ciltacabtagene autoleucel) | BCMA | Multiple myeloma | February, 2022 | NCT03548207 (CARTITUDE-1) | CRS, Neurotoxicity, Delayed Cytopenia |
| Abecma (Idecabtagene vicleucel) | BCMA | Multiple myeloma (relapsed/refractory after ≥2 prior therapies) | March, 2021 | NCT03361748 (KarMMa) | CRS, Neurotoxicity |
Manufacturing CAR T cell therapies presents notable challenges. In lymphogenic and heavily pretreated patients, T cell collection is often difficult, and the prolonged production timeline frequently necessitates bridging chemotherapy.21 Furthermore, the autologous nature of these therapies entails substantial costs and intricate logistics, hindering widespread accessibility and clinical implementation.4 The integration of chemokine receptors such as C-X-C chemokine receptor type 3 (CXCR3) or C-C chemokine receptor type 4 (CCR4) into CAR-Tregs significantly enhances their trafficking to inflamed tissues, thereby augmenting their immunosuppressive function.22,23 In contrast to conventional CAR T cells, CAR-Tregs demonstrate a superior safety profile, with no reported instances of tumor lysis syndrome or cytokine release syndrome. Furthermore, the incorporation of safety mechanisms, including suicide genes or reversible “OFF” switches, enables precise control over their activity and facilitates their selective elimination when required.22 CAR-Tregs, characterized as CD4+ Helios+ T cells expressing immunosuppressive markers such as FOXP3 and CD25, play an essential role in modulating immune responses during cancer immunotherapy, particularly in the context of CD19-targeted CAR T cell therapy for large B-cell lymphoma. Detected as early as day 7 post-infusion, these cells are associated with reduced neurotoxicity and delayed disease progression, largely due to their regulatory effect on CAR T cell expansion, especially within the CD8+ subset. Notably, integrating CAR-Treg frequency with lactate dehydrogenase levels, a surrogate marker of tumor burden, improves the prediction of clinical outcomes. This combination offers a valuable biomarker for refining therapeutic strategies and managing toxicity in patients with large B-cell lymphoma.24
In contrast to effector CAR T cells that promote immune activation, CAR-Tregs utilize an immunosuppressive mechanism to maintain immune balance and control pathological responses.25 This key distinction underpins their therapeutic promise in settings such as transplantation and autoimmune diseases. However, advancing their clinical application remains challenging, particularly due to the lack of standardized protocols for activation, expansion, and the reliable establishment of a stable regulatory phenotype.22
Tregs and the emergence of CAR-Treg therapy in autoimmune disease
Tregs are a specialized subset of CD4+ T lymphocytes characterized by the expression of the transcription factor FOXP3, and they play an indispensable role in maintaining immune tolerance and homeostasis.26,27 Tregs suppress autoreactive immune cells and prevent aberrant immune activation through multiple mechanisms: secretion of immunoregulatory cytokines such as TGF-β, IL-10, and IL-35; engagement of CTLA-4 to inhibit co-stimulatory signaling; and metabolic disruption via high-affinity CD25-mediated sequestration of IL-2, thereby depriving effector T cells of critical growth signals.28 In autoimmune diseases, diminished Treg frequency or functional impairment permits the unchecked activity of autoreactive lymphocytes, culminating in chronic inflammation and tissue damage.29 Additionally, Tregs can modulate antigen-presenting cells by downregulating co-stimulatory molecules such as CD80 and CD86, limiting their ability to prime autoreactive T cells.30 CAR-Tregs have emerged as a next-generation, precision immunotherapy for autoimmune and inflammatory diseases. By equipping Tregs with synthetic CARs that target disease-relevant autoantigens, these engineered cells can home to sites of inflammation and re-establish immune tolerance in a highly localized and antigen-specific manner.31,32 Preclinical studies in models such as experimental autoimmune encephalomyelitis (EAE)—a surrogate for MS—and colitis have demonstrated their capacity to suppress inflammation and reverse disease phenotypes.33 In contrast to conventional immunosuppressants, which broadly suppress the immune system, CAR-Tregs provide focused modulation of pathogenic immune responses while preserving overall immune competence.34 Despite the promise of CAR-Treg therapy, several challenges persist, including the optimization of CAR design, enhancement of in vivo persistence, and mitigation of phenotypic instability. Phenotypic instability in CAR-Treg therapy is a major challenge since Tregs may lose their suppressive function and convert into effector T cells. The extent of this instability varies with CAR design, as high-affinity CARs and certain signaling domains (e.g., 4-1BB) may exacerbate instability and promote pro-inflammatory cytokine production. Strategies to enhance CAR-Treg stability include careful CAR construct selection, use of endogenous Treg populations, optimization of costimulatory signals, and incorporation of safety mechanisms.35 Nevertheless, ongoing clinical trials in conditions such as pemphigus vulgaris and T1D mark a significant shift from broad immunosuppression toward antigen-specific immune reprogramming.36,37 Advances in genomics, synthetic biology, and biomaterial engineering are poised to refine CAR-Tregs technology further, enhancing their safety, stability, and therapeutic precision.38 Collectively, these innovations are paving the way for a new era in the treatment of autoimmune and inflammatory diseases—one defined by durable, localized, and highly specific immune regulation.39
Polyclonal Treg cells as cellular therapeutics
Polyclonal Treg cells, defined by their diverse T-cell receptor (TCR) repertoire, have shown significant therapeutic promise in various preclinical disease models and early-phase clinical studies. They have demonstrated effective immune modulation and the ability to promote immune tolerance in murine models of GvHD,40,41 SLE, EAE, colitis, and T1D; adoptive transfer of polyclonal Tregs led to reduced pathology, diminished immune cell infiltration, and overall disease amelioration.42 Human polyclonal Tregs have also demonstrated protective effects in xenogeneic GvHD models when transferred into immunodeficient mouse strains. These include Rag2−/− γc−/− mice deficient in both recombination-activating gene 2 and the common gamma chain (Il2rg) and severe combined immunodeficient mice, underscoring their capacity to modulate pathogenic immune responses across species barriers.43
The phenotypic stability and purity of the cell product are critical factors in the success of polyclonal Treg therapy. Enrichment of naïve Treg subsets, identified by markers such as CD62L in mice and CD45RA in humans, has been associated with sustained FOXP3 expression, driven by epigenetic stability, ultimately enhancing their functional performance in vivo.44 To generate therapeutically relevant quantities of polyclonal Tregs, standard expansion protocols commonly employ anti-CD3/CD28 stimulation in the presence of high-dose IL-2.45 The safety and efficacy of these expanded Tregs have been investigated in multiple Phase 1 and 2 clinical trials targeting a variety of conditions, including T1D (ISRCTN06128462, NCT01210664), GvHD (NCT01660607, NCT00602693, NCT02423915), SLE (NCT02428309), Crohn’s disease (NCT03185000), and COVID-19 (NCT04468971). Particularly noteworthy is a Phase 1/2 study of Orca-T, a donor-derived, polyclonal Treg-enriched product administered within 60 h of collection without ex vivo expansion, which demonstrated a markedly reduced incidence of both acute and chronic GvHD (4–5%) compared to historical rates of 30%–40%. These encouraging outcomes have prompted the initiation of a Phase 3 randomized clinical trial comparing Orca-T with standard-of-care in patients with acute leukemia undergoing allogeneic hematopoietic stem cell transplantation (NCT05316701).43 Despite significant progress, the therapeutic outcomes of polyclonal Treg therapy remain variable, primarily due to inconsistencies in isolation and expansion protocols employed across different studies.46 In addition, the absence of antigen specificity in polyclonal Tregs can constrain their therapeutic efficacy in specific settings, thereby driving the advancement of engineering approaches aimed at improving their target specificity and functional precision.47,48
Antigen-specific Tregs: Enhancing precision in Treg-based immunotherapy
Although polyclonal Treg cells have shown safety and therapeutic potential across various preclinical and clinical contexts, their lack of antigen specificity can result in systemic immunosuppression, often requiring high cell doses to achieve efficacy. The advent of CAR technology, originally developed to enhance T cell targeting in cancer immunotherapy, has introduced a transformative strategy when applied to Tregs. However, employing generated Tregs for the treatment of autoimmune disorders presents a significant drawback. This occurs because induced Tregs are polyspecific, meaning they might inhibit beneficial immune responses when administered to a patient. The initial phase involved generating induced Tregs that precisely targeted a certain autoantigen. Molecular engineering is essential for the development of CAR-equipped Tregs. CARs represent a viable approach for redirecting the specificity of Treg cells.16
By equipping Tregs with CARs, their immunosuppressive activity can be precisely directed toward specific antigens or tissues. This approach synergizes the natural regulatory function of Tregs with the targeting accuracy of CARs, enhancing their specificity, potency, and durability. CAR-Tregs can be tailored to recognize disease-relevant antigens in autoimmune disorders, donor-specific antigens in transplantation, or tissue-restricted antigens in inflammatory diseases, enabling localized immune regulation while minimizing the systemic effects commonly seen with conventional immunosuppressive therapies.2,3
Engineering of CAR-Tregs for autoimmune disease therapy
TCR-engineered Tregs face limitations due to their dependence on MHC-restricted antigen recognition, which increases the risk of mispairing between endogenous and introduced TCR chains, thereby complicating their clinical utility. Additionally, their survival and suppressive function remain highly dependent on IL-2.49 In contrast, CAR-Tregs offer several compelling advantages. Their MHC-independent antigen recognition broadens their applicability, particularly in the contexts of transplantation and autoimmune disease.2 CAR-Tregs also display enhanced homing to antigen-expressing tissues, reduced IL-2 dependency, and the ability to proliferate independently, thereby improving their therapeutic efficacy.3 A foundational preclinical study in 2008 demonstrated that CAR-Tregs could localize to inflamed colonic tissue and suppress effector T cell activity in an antigen-specific, MHC-unrestricted manner, resulting in notable improvement of colitis.50 Emerging evidence suggests that CAR-Tregs surpass TCR-engineered Tregs in their ability to prevent GvHD, exhibiting greater suppressive potency and more consistent therapeutic outcomes.51 Engineering Tregs with CARs has emerged as a compelling strategy for the treatment of autoimmune diseases. This innovative approach leverages the intrinsic immunosuppressive function of CD4+ Tregs while conferring antigen specificity through precise genetic modification, enabling focused immune modulation. Unlike conventional CAR-T cells, which are designed to eliminate malignant targets, CAR-Tregs are programmed to suppress aberrant immune activity at its source. This precision-targeted strategy offers a significant advantage over traditional immunosuppressive therapies by minimizing systemic immune suppression and preserving the body’s ability to combat infections.52,53 The engineering of CARs for Tregs builds upon foundational CAR T cell technology, with specific modifications tailored to the unique functional and phenotypic characteristics of Tregs. A typical CAR construct consists of three key components: an antigen-binding domain, a transmembrane domain, and an intracellular signaling domain.
In the treatment of autoimmune diseases, CAR-Treg therapy enables precise, antigen-specific immune modulation. The process begins with the isolation of peripheral blood mononuclear cells from sources such as thymus, umbilical cord blood, or peripheral blood.54 Tregs are then enriched based on either naïve surface markers (CD4+, CD25high, CD127low, and CD45RA+), which are associated with superior proliferative potential and phenotypic stability, or conventional markers (CD4+, CD25high, CD127low).55,56 CAR introduction is typically performed using viral vectors, including lentiviral, gamma-retroviral, or adeno-associated viral systems. However, interest is growing in non-viral gene delivery approaches, such as the Sleeping Beauty transposon system and clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 genome editing. Notably, CRISPR-engineered CAR-Tregs have shown enhanced durability and functional performance compared to their virally transduced counterparts, highlighting their potential for next-generation cell therapies.3
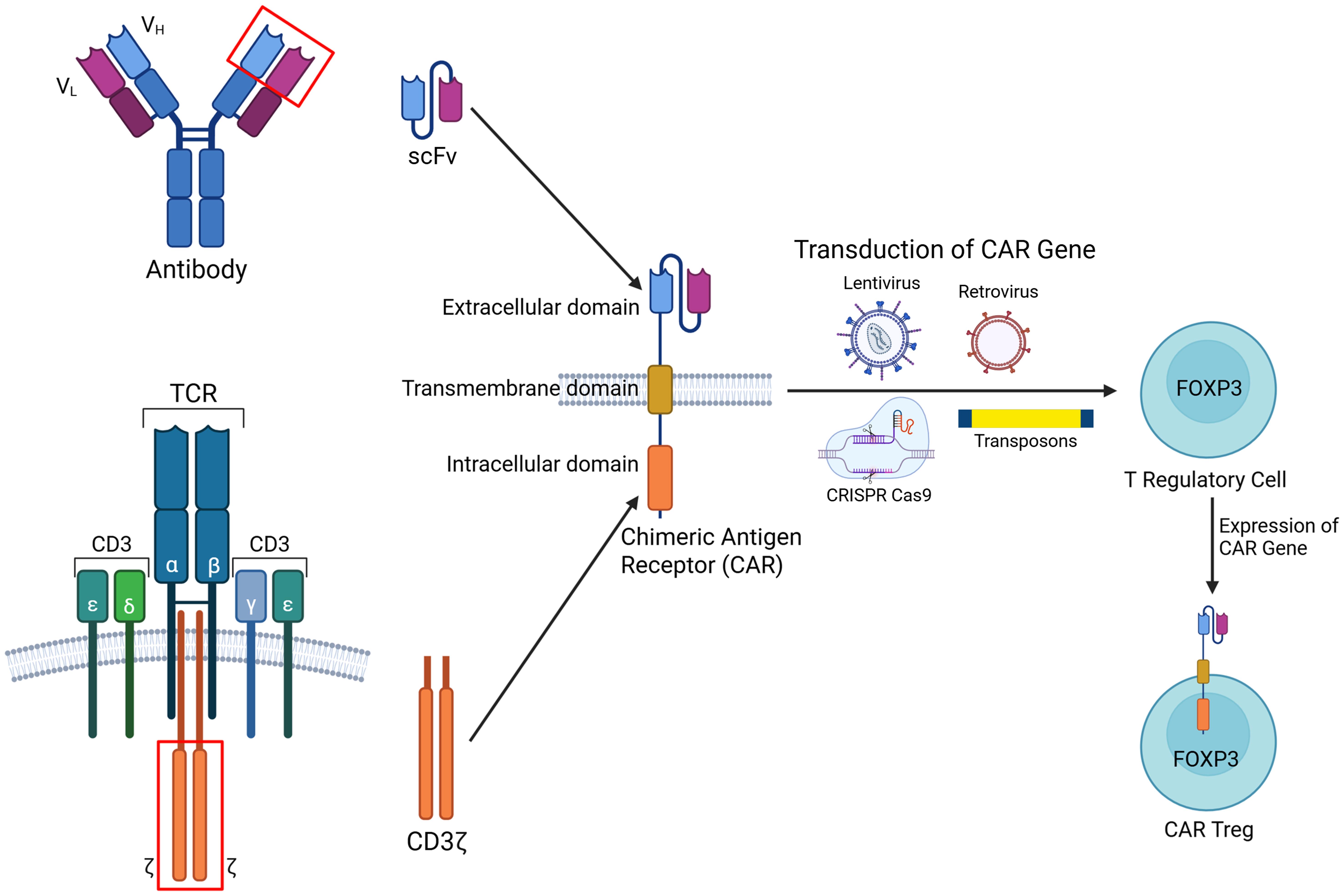
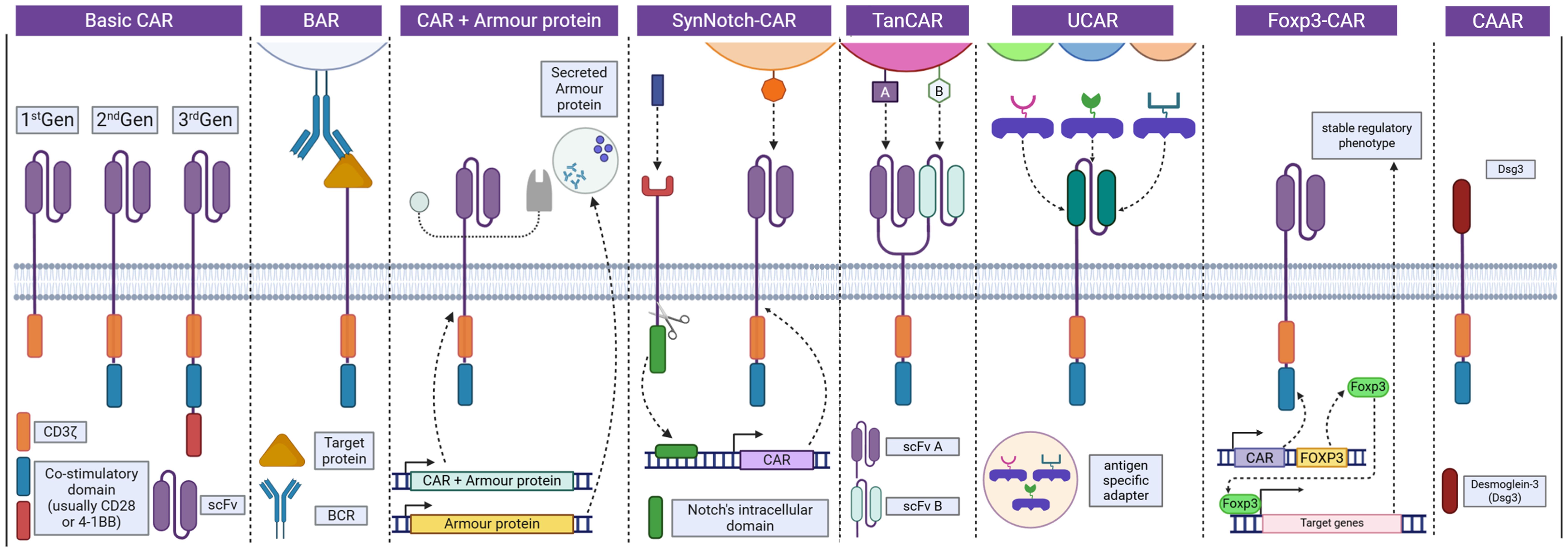
CAR-Treg construction integrates scFv from monoclonal antibodies to ensure specific antigen recognition. These are fused with co-stimulatory domains, such as CD28 or 4-1BB, which are essential for promoting CAR-Treg persistence, functional stability, and immunosuppressive efficacy.57FOXP3, the master transcription factor critical for sustaining the regulatory identity of conventional T cells, can be upregulated through several approaches (Fig. 1). These include viral transduction, homologous directed recombination, Treg-specific demethylated region demethylation, and inhibition of histone deacetylases.22 Emerging CRISPR/Cas9 gene editing technologies hold significant potential to reinforce Treg stability by upregulating FOXP3 expression. By preserving the immunosuppressive identity of CAR-Tregs, this strategy reduces the likelihood of their transdifferentiation into pro-inflammatory phenotypes, thereby enhancing therapeutic reliability and safety.58 CAR constructs can be integrated into the cells by CRISPR/Cas9, homologous directed recombination, or by using viral vectors.59Table 2 lists the current preclinical and clinical development of CAR-Treg therapy for autoimmune diseases.32,60–65 Engineered Tregs can be broadly categorized into existing and emerging CAR-Treg platforms. Current designs include conventional CAR-Tregs, B cell-targeting antibody receptor-Tregs, FOXP3-CARs for enhanced phenotypic stability, and universal CAR-Tregs developed for allogeneic applications.33,66 Meanwhile, innovative platforms under investigation include Armor-CARs, designed to enhance suppressive function; Tandem CARs, which enable dual-antigen targeting; and SynNotch-CARs (Fig. 2),22 which provide programmable control over CAR expression.67,68
A chimeric receptor is synthesized by combining the ScFv derived from the variable regions of an antibody and the intracellular signaling domain CD3ζ from the T cell receptor (TCR). The CAR gene is introduced into FOXP3+ T regulatory (Treg) cells using viral (lentiviral or retroviral) or non-viral (transposon systems, CRISPR/Cas9) vectors. The result is a CAR-expressing Treg cell with retained immunosuppressive capacity and targeted antigen recognition. The figure was created with BioRender. CRISPR, clustered regularly interspaced short palindromic repeats; FOXP3, forkhead box protein P3; ScFv, single-chain variable fragment.
CAR-Treg therapies for autoimmune diseases: Preclinical and clinical developments
| Disease | Antigen specificity | Results | Clinical trials | References |
|---|---|---|---|---|
| T1D | Insulin B peptide – MHC class II | Mouse CAR-Tregs prevented adoptive transfer diabetes by BDC2.5 T cells in immunodeficient NOD mice and prevented spontaneous diabetes in wild-type NOD mice | Preclinical | 60 |
| T1D | Insulin B peptide – MHC class II | Mouse InsB:R3-CAR-Tregs protected against spontaneous diabetes in NOD.CD28−/− mice | Preclinical | 61 |
| Multiple sclerosis | Myelin oligodendrocyte glycoprotein (MOG) | MOG-specific CAR-Tregs suppressed CNS inflammation and prevented disease progression in EAE mice modeling multiple sclerosis | Preclinical | 32 |
| Rheumatoid Arthritis | Citrullinated proteins | SBT777101, an autologous regulatory CAR-Treg cell therapy targeting citrullinated proteins, was well tolerated with no serious adverse events in patients with moderate-to-severe rheumatoid arthritis | Phase 1 trial (NCT06201416) | 62 |
| Crohn’s Disease | Interleukin-23 receptor (IL23R) | IL23R-specific CAR-Tregs effectively suppressed intestinal inflammation and restored immune homeostasis in humanized mouse models of Crohn’s disease | Preclinical | 63 |
| Systemic lupus erythematosus (SLE) | CD19 | In a humanized mouse model of SLE, a single infusion of FOXP3-overexpressing, CD19-targeted CAR-Tregs (FOX19 CAR-Tregs) suppressed B cell activity, reduced autoantibody production, and restored immune homeostasis without detectable toxicity | Preclinical | 64 |
| IBD | Flagellin | FliC-CAR-Tregs specifically recognize flagellin, migrate to the colon, and enhance immunosuppression and intestinal barrier integrity in vitro | Preclinical | 65 |
Basic CARs (1st–3rd generation) differ by added co-stimulatory domains. Variants enhance function: BAR uses BCR; CAR+Armor secretes modulators; SynNotch-CAR enables conditional CAR expression; TanCAR targets dual antigens; UCAR uses modular adaptors; Foxp3-CAR ensures a stable Treg phenotype. CAAR displays autoantigen (Dsg3) to selectively target autoreactive B cells.22 Figure was created with BioRender. BAR, B-cell receptor antigens for reverse targeting; BCR, B-cell receptor; CAAR, chimeric autoantibody receptor; UCAR, universal chimeric antigen receptor.
CAR-Tregs: Mechanism of action
CAR-Tregs exert their immunosuppressive effects through a diverse and synergistic array of mechanisms. Upon antigen engagement, they secrete anti-inflammatory cytokines such as IL-10 and TGF-β, fostering a tolerogenic microenvironment that is critical for maintaining immune tolerance.28 Moreover, CAR-Tregs secrete cytotoxic mediators such as granzyme B, enabling the selective elimination of autoreactive effector T cells and thereby reducing inflammation while maintaining protective immunity.69 In contrast, their elevated expression of CTLA-4 suppresses co-stimulatory signals on antigen-presenting cells, effectively limiting the activation of pro-inflammatory T cells.69 Furthermore, through the expression of CD25, CAR-Tregs efficiently sequester IL-2, limiting its availability to effector T cells and thus curbing their expansion while sustaining immune tolerance.70
One of the key advantages of CAR-Tregs is their ability to target disease-specific antigens, such as myelin basic protein in MS or insulin and GAD65 in T1D, enabling precision targeting without the off-target effects seen in traditional treatments. Preclinical studies have demonstrated that CAR-Tregs engineered to recognize myelin-specific antigens successfully reduce neuroinflammation and demyelination in MS models,71 while CAR-Tregs targeting pancreatic islet antigens protect β-cells in T1D, thereby preserving insulin production.55,72,73 In GvHD, CAR-Tregs targeting alloantigens have been shown to reduce tissue damage while maintaining systemic immunity. This demonstrates CAR-Tregs’ capacity to deliver more localized and precise immunosuppression than conventional therapies.74
Therapy-induced targets in autoimmune diseases
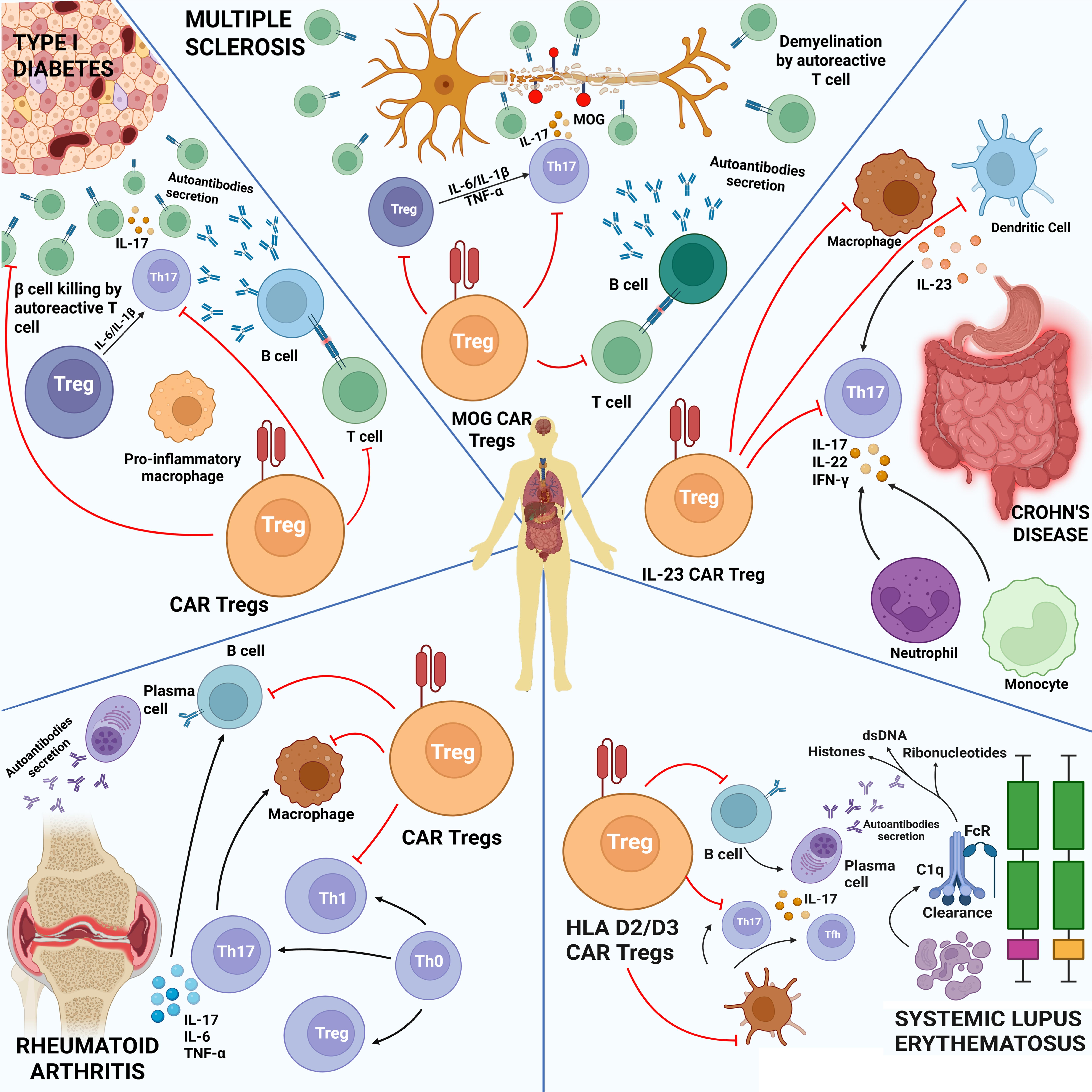
Type 1 diabetes mellitus (T1DM): Tregs in T1DM cause the immune system to attack and destroy pancreatic β-cells.75 To combat autoimmune destruction, CAR-Tregs have been engineered to selectively recognize peptide–MHC class II complexes,60 such as Insulin B10–23:I-Ag7 and p63:I-Ag7, which are predominantly expressed in the pancreas and draining lymph nodes.76 Whereas autoreactive T cells drive the destruction of β-cells (Fig. 3), CAR-Tregs are programmed to suppress this pathogenic response and attenuate proinflammatory macrophage activity. In comparison with polyclonal Tregs, antigen-specific CAR-Tregs demonstrate superior efficacy in restraining the proliferation of diabetogenic T cells such as BDC2.5 and in reducing inflammatory cytokine production in vitro.77,78 In animal models, CAR-Tregs targeting InsB10–23:I-Ag7 delayed the onset of diabetes in non-obese diabetic mice and significantly lowered disease incidence relative to controls. The targeted and stable expression of peptide-MHC complexes at sites of autoimmune activity enhances their therapeutic efficacy.61,79 These results underscore the potential of CAR-Tregs to precisely regulate immune responses in T1DM through antigen-specific intervention.80Rheumatoid arthritis: In Rheumatoid arthritis, compromised Treg function results in elevated levels of proinflammatory cytokines like interferon-γ and tumor necrosis factor-α, intensifying inflammation and promoting joint deterioration.81,82 CAR-Tregs are showing great promise as a therapeutic strategy to re-establish immune tolerance in inflamed joints.9 Ovalbumin-specific Tregs—whether engineered with TCR alone or with combined TCR and FOXP3 transduction83—can effectively diminish antigen-driven immune responses in joints by targeting and suppressing inflammatory T helper 17 (Th17) cells, thereby reducing tissue damage. In rheumatoid arthritis, citrullinated vimentin (CV) has emerged as a critical target, leading to the development of CV-specific CAR-Tregs designed to selectively identify and eliminate CV-positive cells in affected joints.47,84 Through localized immune suppression, these CV-specific CAR-Tregs offer a potential novel treatment avenue for rheumatoid arthritis.82,85,86 To reduce synovial inflammation and protect joint integrity, CAR-Tregs (Fig. 3) act as modulators of aberrant Th17-driven responses,87 autoantibody-secreting B cells, and essential inflammatory mediators like IL-17, IL-6, and TNF-α.88 Targeting CAR-Treg cells may help regulate the immune system in affected tissues of rheumatoid arthritis without using broad immunosuppressive therapy.89SLE: SLE is a multifaceted autoimmune disorder marked by a loss of immune self-tolerance, primarily driven by overactive B cells,90 T cells, and dysregulated innate immune components.91 This complex immune dysfunction triggers widespread inflammation and the generation of harmful autoantibodies, underscoring the systemic impact of the disease.92 Although they require continuous use and pose risks such as infection, modern biologic treatments, including monoclonal antibodies aimed at disease-specific targets, have markedly improved disease control.93 The persistence of autoreactive memory cells, such as plasma cells that continue to generate harmful autoantibodies despite treatment, is a significant problem.94 Given the limitations of conventional treatments, CAR-Tregs—engineered Tregs—have emerged as a promising approach to more precisely re-establish immune tolerance in SLE. By specifically targeting HLA-D2/D3 molecules,95 CAR-Tregs can inhibit autoreactive B cells and plasma cells responsible for pathogenic autoantibody production, while also suppressing proinflammatory cytokines such as IL-17 (Fig. 3).96,97 This targeted strategy avoids the broad immunosuppression associated with traditional therapies, offering the potential for long-term disease control. It reflects a growing shift toward antigen-specific immunomodulation and addresses the limitations of polyclonal Treg therapies, with the goal of achieving sustained remission in SLE patients.47,98
This schematic illustrates how disease-specific CAR-Tregs can modulate immune responses in various autoimmune diseases by suppressing key inflammatory pathways and cell types. In T1D, CAR-Tregs suppress β-cell–targeting T cells and enhance insulin production. In Multiple Sclerosis, MOG-specific CAR-Tregs inhibit demyelinating Th17 responses and B-cell autoantibody production. In Crohn’s Disease, IL-23–specific CAR-Tregs dampen Th17-driven gut inflammation and immune cell recruitment. In Rheumatoid Arthritis, CAR-Tregs restore joint homeostasis by suppressing Th17 cells, macrophages, and cytokine release. In Systemic Lupus Erythematosus, HLA-D2/D3-specific CAR-Tregs reduce autoreactive B-cell activity and immune complex formation, limiting systemic inflammation. The figure was created with BioRender. HLA, human leukocyte antigen; MOG, myelin oligodendrocyte glycoprotein; Th17, T helper 17.
Crohn’s disease: Crohn’s disease is a chronic autoimmune disorder characterized by transmural inflammation that can involve any segment of the gastrointestinal tract.99 The disease arises from a dysregulated immune response, compounded by intricate and imbalanced interactions between the host immune system and the gut microbiota.100 These maladaptive immune-microbial relationships drive ongoing inflammation and intestinal damage, underpinning the progressive nature of Crohn’s disease.101,102 Proinflammatory Th17 cells are key contributors to the pathogenesis of Crohn’s disease, secreting cytokines such as IL-17, IL-22, and interferon-gamma (IFN-γ) that drive intestinal inflammation and tissue destruction.103 This inflammatory cascade is further amplified by dendritic cells and macrophages, which support Th17 cell activation through IL-23 production. CAR-Treg therapies, particularly those targeting the IL-23 receptor (IL-23R), offer a targeted approach to modulating this immune dysregulation.104 IL-23R CAR-Tregs are designed to home to inflamed intestinal sites, where they suppress Th17-mediated responses, downregulate proinflammatory cytokine release, and inhibit the recruitment of neutrophils and monocytes. By reestablishing immune tolerance in a highly specific and localized manner, IL-23R CAR-Tregs represent a compelling therapeutic alternative to conventional immunosuppressive therapies (Fig. 3), with the potential to deliver more effective and durable disease control.3MS: CAR-Treg-cell therapy is gaining recognition as a promising, targeted therapeutic approach for MS—a chronic autoimmune neurodegenerative disease driven by autoreactive T cells and the formation of pathogenic autoantibodies that lead to demyelination and neurological damage.105 More targeted approaches are required because traditional treatments, such as broad immunosuppressants and B-cell-depleting monoclonal antibodies, can cause systemic immune suppression and adverse effects.106 CAR-Tregs designed to detect myelin antigens, such as myelin oligodendrocyte glycoprotein,107 have demonstrated therapeutic potential in light of the functional deficiencies in Tregs in MS patients, including increased IFN-γ and decreased IL-10 production.108,109 Antigen-specific CAR-Tregs reduce proinflammatory responses and autoantibody release,110 reducing neuroinflammation through IL-10-dependent mechanisms,111 according to preclinical research conducted on EAE models.112–114 Notably, myelin basic protein (myelin oligodendrocyte glycoprotein)-targeting altered Tregs effectively relocate to the central nervous system, suppress effector T cells (Fig. 3), and lessen disease severity.34,115 Interest in creating CAR-Treg treatments to lower MS-associated morbidity has increased as a result of these developments.
CAR-Tregs clinical trials
Preclinical studies and early-phase clinical trials have demonstrated the safety and promising therapeutic efficacy of CAR-Treg therapy in fostering immune tolerance. In particular, CD45RA+ CD19 CAR-Tregs have shown superior functionality, marked by sustained and elevated expression of key regulatory markers such as FOXP3, Helios, and CTLA-4—surpassing the performance of their CD45RO+ counterparts.22,116 Currently, three clinical trials are actively investigating monospecific CAR-Tregs in human subjects, representing a significant milestone for the field. At present, three clinical trials are actively testing monospecific CAR-Tregs in humans, marking a pivotal milestone in the advancement of the field (Table 3). However, widespread clinical success will depend on continued innovation, including the development of CAR-Tregs capable of targeting multiple antigens to enhance both therapeutic specificity and persistence. Furthermore, the incorporation of ‘armor proteins’ offers a compelling strategy to reinforce functional stability, although comprehensive preclinical evaluation is necessary prior to clinical application.22 Armor proteins include pro-inflammatory cytokines and antibody-like fragments that boost T-cell activity, cancer cell–killing ability, and infiltration.
CAR-Tregs therapies: Registered clinical trials
| Clinical trial | Population characteristics | Specifications | Phase of trial | Status | Results | Primary outcomes | Study duration | Clinical trials (NCT ID) |
|---|---|---|---|---|---|---|---|---|
| Safety and tolerability of CAR-Treg therapy in living donor kidney transplant patients (STEADFAST) | Living donor renal transplant recipients | HLA-A2 CAR-Tregs, autologous | Phase I/II | Active, not recruiting | Submitted but not available on clinicaltrials.gov | This study aims to evaluate the safety and tolerability of TX200-TR101 and its impact on the transplanted kidney in living donor kidney transplant recipients | 28 days post infusion | NCT04817774 |
| Safety and clinical activity of QEL-001 in A2-mismatch liver transplant patients (LIBERATE) | A2-mismatch liver transplant patients | HLA-A2 CAR-Tregs, autologous | Phase I/II | Recruiting | No results posted | Long-term safety and Tolerability | 28 Days post infusion; from the day of infusion through Week 82 and up to 15 years post-infusion | NCT05234190 |
| Allogeneic CD6-CAR-Tregs for the treatment of patients with chronic graft versus host disease following allogeneic hematopoietic cell transplantation | Patients with chronic graft versus host disease (cGVHD) after allogeneic hematopoietic cell transplantation | Allogeneic CD6 CAR-Tregs | Phase I | Recruiting | No results posted | Dose-limiting toxicity | From infusion of CD6-CAR- Tregs to day +28 | NCT05993611 |
| Long-term follow-up of patients who have received an autologous antigen-specific CAR-Treg therapy (TX200-TR101) | Kidney transplant recipients (18–72 years) | Autologous antigen-specific CAR-Tregs | Phase I/IIa | Enrolling by invitation | No results posted | Safety and tolerability of TX200-TR101 infusion assessed by overall survival and incidence of serious adverse events (SAEs) per CTCAE v5.0 | Up to 15 years post-infusion | NCT05987527 |
| First-in-human trial of CAR19 regulatory T cells (CAR19-tTreg) in adults with relapsed/refractory CD19+ B acute lymphoblastic leukemia | Adults with relapsed/refractory CD19+ B-ALL after failure of standard therapies | Allogeneic CAR19 regulatory T cells (CAR19-tTreg) | Phase I/II | Withdrawn (Replaced with a new IND and protocol) | No results posted | Measure CAR19-tTregs efficacy and dose standardization | 28 days after CAR19-tTregs administration | NCT05114837 |
| Study of single doses of sbt777101 in subjects with rheumatoid arthritis (Regulate-RA) | Subjects with Rheumatoid Arthritis | SBT777101 | Phase I | Recruiting | No results posted | Incidence and nature of dose-limiting toxicities (DLTs) | Day of treatment to end of follow-up (48 weeks). Day of treatment to end of DLT evaluation period (28 days) | NCT06201416 |
Advancements in CAR-Treg therapy
Recent advancements in CAR-Treg therapies have significantly expanded their therapeutic potential in the treatment of autoimmune diseases, transplant rejection, and other immune-mediated disorders. A key development is the enhancement of antigen specificity, enabling CAR-Tregs to be engineered to recognize a broader array of disease-relevant targets, thereby improving their efficacy.117 Additionally, the advent of universal CAR platforms marks a transformative step forward, offering modular systems with interchangeable adapter molecules that allow CAR-Tregs to flexibly and precisely target diverse tissue antigens while preserving their immunoregulatory function and promoting immune tolerance.22 The development of B cell-targeting antibody receptor-Tregs has shown promising results, particularly in controlling severe allergic reactions and modulating excessive antibody responses. Additionally, the CD28 co-stimulatory domain is essential for maintaining CAR-Treg stability and function, helping to prevent premature exhaustion and ensuring long-lasting therapeutic efficacy. The addition of ‘armor’ proteins like IL-2,118 TGF-β, and IL-10,119 has further strengthened the suppressive function, durability, and survival of CAR-Tregs, enhancing their capacity to sustain immune tolerance.
Innovations in orthogonal IL-2 receptors enable the selective expansion of CAR-Tregs, offering precise regulation of cell growth without disrupting the body’s natural immune signaling pathways. Furthermore, CRISPR-Cas9 technology has enabled the precise insertion of CAR constructs into the TRAC locus, ensuring consistent CAR expression while reducing risks such as tonic signaling and Treg exhaustion. These advancements are revolutionizing CAR-Treg therapies, enhancing their stability, efficacy, and adaptability as powerful tools in precision medicine.120 CAR-Tregs and chimeric autoantibody receptor (CAAR)-Tregs offer cutting-edge therapeutic approaches for autoimmune diseases by precisely targeting specific antigens to inhibit autoreactive immune responses. In Pemphigus Vulgaris, preclinical research has shown that CAR-Tregs directed against desmoglein 3 (DSG3) can significantly decrease anti-DSG3 antibody production, presenting a non-cytotoxic treatment option. Ongoing clinical trials, such as NCT04422912, are assessing the efficacy of CAAR-Tregs targeting DSG3 in promoting sustained disease remission.20 In Myasthenia Gravis, CAAR-Tregs designed to target muscle-specific tyrosine kinase autoantibodies show promise in selectively suppressing antigen-specific B-cell responses. Ongoing clinical trials, including NCT05451212, are evaluating their effectiveness in improving patient outcomes.20 Similarly, in Neuromyelitis Optica Spectrum Disorders, CAR-Tregs directed against CD19 and CD20 are being evaluated in clinical trials (NCT03605238), aiming to reduce AQP4-IgG levels and alleviate clinical symptoms.20 In idiopathic inflammatory myositis, CAR-Tregs targeting CD19 and CD7 are engineered to regulate B-cell activity and alleviate muscle tissue damage. Likewise, in SLE, CAR-Tregs aimed at CD19 are being developed to deplete autoreactive B cells and diminish autoantibody production.20 Preclinical research on T1D utilizing CAAR-Tregs targeting the I-Ag7-B:9–23 complex has shown promising results in delaying disease onset.20 CAR-Tregs hold significant promise not only for autoimmune diseases but also in transplantation, where they may effectively prevent graft rejection and control GvHD.64 The antigen-specific suppressive capabilities of CAR-Tregs significantly enhance immune tolerance toward transplanted tissues, thereby improving both the safety and efficacy of transplantation therapies.121 Furthermore, their capacity to restore immune homeostasis without inducing broad immunosuppression positions CAR-Tregs as a valuable adjunct in the field of transplantation medicine.50
The development of CAR-Tregs and CAAR-Tregs faces several critical challenges. Immunogenicity remains a major concern, as immune responses against CAR components could potentially aggravate autoimmune conditions.5,122 Furthermore, manufacturing complexities, including the reliable incorporation of autoantigens and maintenance of Treg regulatory function, continue to hinder clinical translation and large-scale application.20,123 In conclusion, CAR-Tregs and CAAR-Tregs hold significant promise as next-generation therapies for autoimmune diseases, offering the potential for targeted and durable immune regulation. Nonetheless, their clinical success hinges on addressing critical challenges such as immunogenicity, efficient and scalable manufacturing, and refined antigen specificity. Resolving these issues will be essential to ensure their safety, efficacy, and broad accessibility in real-world therapeutic settings.124
Challenges in CAR-Treg therapy
Although CAR-Treg therapy holds considerable promise, its scalability remains limited by the labor-intensive and costly processes required for Treg isolation and expansion, thereby constraining widespread clinical application. Innovations in bioreactor technologies and the development of off-the-shelf allogeneic CAR-Treg products offer potential solutions to these challenges, paving the way for broader accessibility.71 Compared to conventional flask-based culture, bioreactors provide an automated system that maintains optimal nutrient supply, oxygenation, and waste removal, in addition to addressing quality control issues. Ongoing clinical trials are currently assessing the safety and efficacy of CAR-Tregs in autoimmune conditions such as T1D and pemphigus vulgaris, providing critical insights into their therapeutic potential in human patients.52
The therapeutic efficacy of Tregs depends on their stability and plasticity. Non-engineered Tregs may transform into proinflammatory Th17 cells under specific conditions, which can compromise their immunosuppressive capabilities.3 Engineered Tregs, such as CAR-Tregs, can achieve enhanced stability through co-transduction with FOXP3 cDNA, which helps maintain their suppressive phenotype and prevents unwanted lineage diversion.3 Certain CAR-Tregs, particularly those incorporating 4-1BB costimulatory domains, may exhibit reduced stability; however, this limitation can be mitigated through adjunctive therapies such as mTOR inhibitors, which help reinforce their regulatory function.3 In contrast, A2-CAR-Tregs maintain high expression of Treg markers and stable epigenetic features, ensuring robust suppressive function and enhanced targeting capabilities.2 Overall, CAR-Tregs demonstrate enhanced stability, antigen specificity, and immunosuppressive capacity compared to polyclonal Tregs, positioning them as a highly promising approach for inducing targeted immune tolerance.
Research underscores the critical role of the CD28 costimulatory domain in boosting CAR-Treg functionality for effective immune suppression. However, challenges such as ligand-independent CAR tonic signaling, which may impair Treg performance, must be resolved to preserve their suppressive ability.51 Despite these hurdles, both preclinical and clinical data highlight the safety and effectiveness of CAR-Tregs compared to traditional CAR-T cells (CAR-Tconvs). By selectively suppressing immune responses in lymphoid organs—where antigen presentation and antibody production take place—CAR-Tregs can interrupt the pathological immune activation that drives autoimmune diseases, all while minimizing the risk of systemic immunosuppression.64
Transfecting CARs into Tregs is commonly achieved using viral vectors, such as lentiviruses or retroviruses. However, these approaches risk insertional mutagenesis from DNA integration, which may lead to neoplasia.125 To improve the safety of CAR-Treg therapies, guided transgene delivery systems offer a promising alternative, enabling safer and more natural CAR expression, for example, by linking the transgene to the TCR promoter while knocking out the TCR.126,127 Non-viral methods, such as liposomes and electroporation, are being explored, but their efficiency remains limited.128 Currently, CAR-Treg therapies rely on autologous CD4+ Tregs, which may be less effective in patients with autoimmune diseases due to Treg dysfunction.129,130 Gene editing to remove MHC-related genes could allow the use of allogeneic Tregs, addressing the challenges of defective autologous Tregs in autoimmune conditions.131
Targeting cells to the specific locations of autoimmune inflammation, such as the synovial joints in rheumatoid arthritis or the pancreatic islets in T1D, is a crucial improvement to CAR-Treg treatment. Following chemokine gradients like C-C motif chemokine ligand 19 (CCL)19 / C-C motif chemokine ligand 21 (CCL21), C-C motif chemokine ligand 17 (CCL17) / C-C motif chemokine ligand 22 (CCL22), C-X-C motif chemokine ligand 9/10/11 (CXCL9/10/11), and native T cells use chemokine receptors like CCR7 to move into lymphoid tissues and CXCR3, CCR4, and CCR8 to migrate into peripheral inflammatory regions.132 The improved localization, retention, and immunosuppressive action at target tissues are demonstrated by preclinical models of CAR T cells designed to co-express receptors such as CCR7 for lymph node homing and CXCR3 or CCR4 for peripheral movement. CAR-Tregs can accomplish targeted, site-specific immune regulation by utilizing such receptor-directed homing techniques, which increases therapeutic potency while lowering systemic effects.133
Limitations
Despite the growing evidence and encouraging recent progress for CAR-Treg therapy in autoimmune diseases and transplantation, this review has certain limitations. First, most of the available evidence is derived from preclinical murine studies, which may not fully capture the complexity of human immune responses, leaving translational efficacy and safety of CAR-Tregs uncertain. Second, clinical trial data on CAR-Tregs are still sparse, and most ongoing studies are in early-phase evaluation, limiting the ability to draw definitive conclusions on long-term outcomes. Third, the heterogeneity of autoimmune diseases complicates the generalization of CAR-Tregs’ therapeutic potential across diverse pathogenic contexts. Finally, this review has primarily focused on scientific and translational perspectives, with less emphasis on economic, ethical, and regulatory considerations, all of which will be crucial for the real-world adoption of CAR-Tregs therapies.
Future implications
CAR-Treg therapy is rapidly emerging as a groundbreaking strategy for the treatment of autoimmune and inflammatory diseases. Originally pioneered for controlling transplant rejection and GvHD, its therapeutic horizon has expanded to encompass complex disorders such as rheumatoid arthritis, SLE, T1D, MS, and ocular conditions like uveitis.41 By enabling precise, antigen-specific immune suppression, CAR-Tregs offer the unique advantage of long-lasting immune modulation without the collateral damage often associated with broad immunosuppressive therapies. This precision holds the potential to significantly reduce—or even eliminate—the need for lifelong immunosuppressive drugs, thereby lowering the risk of opportunistic infections, malignancies, and other systemic complications.
Recent advancements in CAR design have propelled the field forward. The integration of bispecific CARs, universal adapter-based systems, and sophisticated costimulatory domains such as CD28 has markedly improved the specificity, stability, and functional potency of CAR-Tregs.22 Moreover, cutting-edge genetic engineering, such as the introduction of IL-10-secreting CAR-Tregs or the disruption of exhaustion markers like programmed cell death protein 1, is enhancing both phenotypic stability and suppressive durability.134 Innovations in delivery strategies, such as intravitreal administration for localized autoimmune conditions, are opening new therapeutic avenues. Concurrently, the rise of gene-editing technologies like CRISPR and non-viral delivery platforms is facilitating the development of both personalized and allogeneic “off-the-shelf” CAR-Treg products. These off-the-shelf platforms not only offer cost-effective scalability but also promise greater global accessibility—key to making this therapy equitable across diverse healthcare systems.
Despite these strides, important challenges remain. Achieving precise antigen targeting, maintaining long-term Treg stability in inflammatory environments, and scaling manufacturing processes for clinical-grade CAR-Tregs are critical for broader clinical translation. Overcoming these hurdles will be essential to fully unlock the therapeutic promise of this cell-based platform. Looking ahead, CAR-Tregs are poised to redefine the landscape of immunotherapy—not just for autoimmune disease, but also in transplantation medicine and chronic inflammatory conditions. Their ability to restore immune tolerance in a tissue-specific, durable, and non-toxic manner represents a paradigm shift in how we approach immune dysregulation. In summary, CAR-Treg therapy is not just a therapeutic innovation, but a foundational shift toward precision immune modulation. As advancements in CAR engineering, gene editing, and manufacturing continue to evolve, CAR-Tregs are increasingly positioned as the next frontier in immunotherapy, offering tailored, safe, and effective solutions for some of the most challenging immune-mediated diseases.
Conclusions
Building on the clinical success of CAR-T cell therapy, CAR-Treg therapy has emerged as a powerful adaptation of this platform for immune modulation. Early clinical trials such as STEADFAST (NCT04817774) and LIBERATE (NCT05234190) have demonstrated the safety and feasibility of HLA-A2–specific CAR-Tregs in transplantation, while studies like SBT777101 for rheumatoid arthritis highlight its broader therapeutic promise. These pioneering efforts confirm that CAR-Tregs can induce targeted and durable immune tolerance with minimal systemic toxicity. The integration of gene-editing tools, universal CAR platforms, and bioreactor-based expansion systems will be pivotal for CAR-Tregs’ transition from experimental therapy to a clinically transformative tool—redefining precision immunotherapy for autoimmune diseases and transplant medicine.
Declarations
Acknowledgement
The authors Sunil Babu Gosipatala, Abhijit Prasad Mishra, Vikram Singh Meena, and Pragya Ukey gratefully acknowledge the support of Babasaheb Bhimrao Ambedkar University, Lucknow. Manish Kumar gratefully acknowledges the support of Mahindra University, Hyderabad.
Funding
None.
Conflict of interest
The authors have no other conflicts of interest related to this publication.
Authors’ contributions
Conception and design, supervision, visualization, manuscript review and editing (SBG), data collection, analysis, drafting of the original manuscript (APM), drafting and critical revision of the manuscript (VSM), drafting of the original manuscript and figure preparation (PU), supervision, visualization, manuscript writing, review, and editing (MK). All authors have read and approved the final manuscript.

 Author information
Author information