Introduction
Atherosclerotic cardiovascular disease (ASCVD) occurrences, such as strokes, heart attacks, and death, are specific examples of conditions encompassed by the broader term cardiovascular disease (CVD). Coronary artery disease (CAD) is among the other conditions affecting the heart and circulatory system.1 Worldwide, CVD has consistently been the leading cause of death, accounting for 17.8 million deaths in 2017.2 This number is projected to exceed 23.6 million by 2023. In the US, about one-third of deaths have CVD as their primary cause.3 Atherosclerosis, which involves the accumulation of fat and cholesterol in the coronary arteries, is associated with CAD. Globally, CAD is the foremost cause of death; no neighborhood, nation, or ethnic group is exempt. One in two males and one in three females over 40 may experience a coronary event at some point in their lives.4 In patients with CAD, proprotein convertase subtilisin/kexin type 9 (PCSK9) levels have been found to positively correlate with inflammatory markers, including white blood cell count, fibrinogen, and high-sensitivity C-reactive protein. These markers are known to contribute to the development and severity of atherosclerosis. PCSK9 levels were independently associated with white blood cell count, lymphocyte and neutrophil counts, fibrinogen, and high-sensitivity C-reactive protein, even in patients not receiving lipid-lowering therapy. These findings suggest that PCSK9 may be involved in vascular inflammation, suggesting that PCSK9 inhibitors may have anti-inflammatory benefits in CAD, although further research is needed to determine the extent to which this effect is independent of low-density lipoprotein (LDL) cholesterol (LDL-C) lowering.5
Currently, the most widely prescribed medications for lowering cholesterol are statins. Statins typically lower LDL-C by approximately 27% and reduce ASCVD occurrences by 20–40%.6 Additional treatment options are necessary for people who do not achieve sufficient LDL-C reduction or who are intolerant to statin therapy.7,8
This narrative review systematically discusses the role of PCSK9 inhibitors in ASCVD management, including their biological effects, clinical efficacy, safety profile, cost-effectiveness, and potential future uses beyond lipid-lowering.
Importance of cholesterol management in CAD
According to the cholesterol hypothesis, increasing blood cholesterol levels raises the risk of ASCVD, while lowering cholesterol reduces that risk.9 The most thoroughly researched modifiable risk factor linked to ASCVD is LDL.10 LDL receptors (LDLRs), found on the cell membranes of hepatocytes and other cells, play a key role in cholesterol uptake.11 Since LDL-C is undeniably essential to the pathophysiology and progression of atherosclerosis, it has been conclusively demonstrated that elevated LDL-C levels are a modifiable risk factor contributing to the development of CVD.10
PCSK9: Mechanism and function
Biological role of PCSK9
New classes of hypolipidemic medications could play a major role in reducing residual risk for atherosclerotic CAD, considering the ongoing reduction in recommended LDL-C targets, the low rate of goal achievement, and limited adherence to therapy.12 Statins, bempedoic acid, ezetimibe, bile acid sequestrants, fibrates, PCSK9 inhibitors, niacin, and n-3 fatty acids (e.g., icosapent ethyl) are currently available medications that decrease cholesterol.9
PCSK9 was initially identified in the early 2000s and subsequently recognized as a key regulator of cholesterol metabolism.1 It was discovered that the liver is the primary site of PCSK9 production, which is then released into the bloodstream and antagonizes cellular LDLR.13 PCSK9 causes the liver’s LDLR to be degraded in the lysosome, which reduces LDL-C removal from the bloodstream.1 PCSK9, also called neural apoptosis-regulated convertase 1, is a major modulator of hepatic LDLR availability.14 In 2003, it was discovered that activating mutations in PCSK9 cause familial hypercholesterolemia.15 Shortly afterward, it was found that deactivating mutations in PCSK9 lower blood LDL-C levels.16 These identical inactivating mutations are strongly protective against CAD, with an 88% reduction in risk.17
In recent years, there have been notable advancements in cardiovascular lipid-lowering treatments with the development of PCSK9 inhibitors.18,19 The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial, which included 27,564 participants, demonstrated that using a PCSK9 inhibitor decreased LDL-C levels and reduced the risk of cardiovascular events, myocardial infarction, and stroke during a two-year period.20
Primarily, PCSK9 inhibitors lowered lipoprotein (a) and apolipoprotein B, but they also effectively lowered LDL-C and other atherogenic lipoproteins. In high-risk individuals, PCSK9 monoclonal antibodies decreased ASCVD events and improved imaging markers, indicating coronary atherosclerotic plaque stability after a short treatment period (less than three years follow-up).12 Physicians prescribe PCSK9 inhibitors to reduce LDL-C levels. These inhibitors can be administered alongside statins or ezetimibe, a drug that inhibits cholesterol absorption in the small intestine.12 Adding PCSK9 inhibitors to patients already taking statins results in a significant reduction in LDL-C levels and a gradual decline in ischemic cardiovascular events.21 ASCVD events have decreased in PCSK9 inhibitor clinical trials, especially in individuals with recent multivessel CAD, acute coronary syndrome, or peripheral arterial disease (see Fig. 1).22
PCSK9-mediated reduction of LDLR
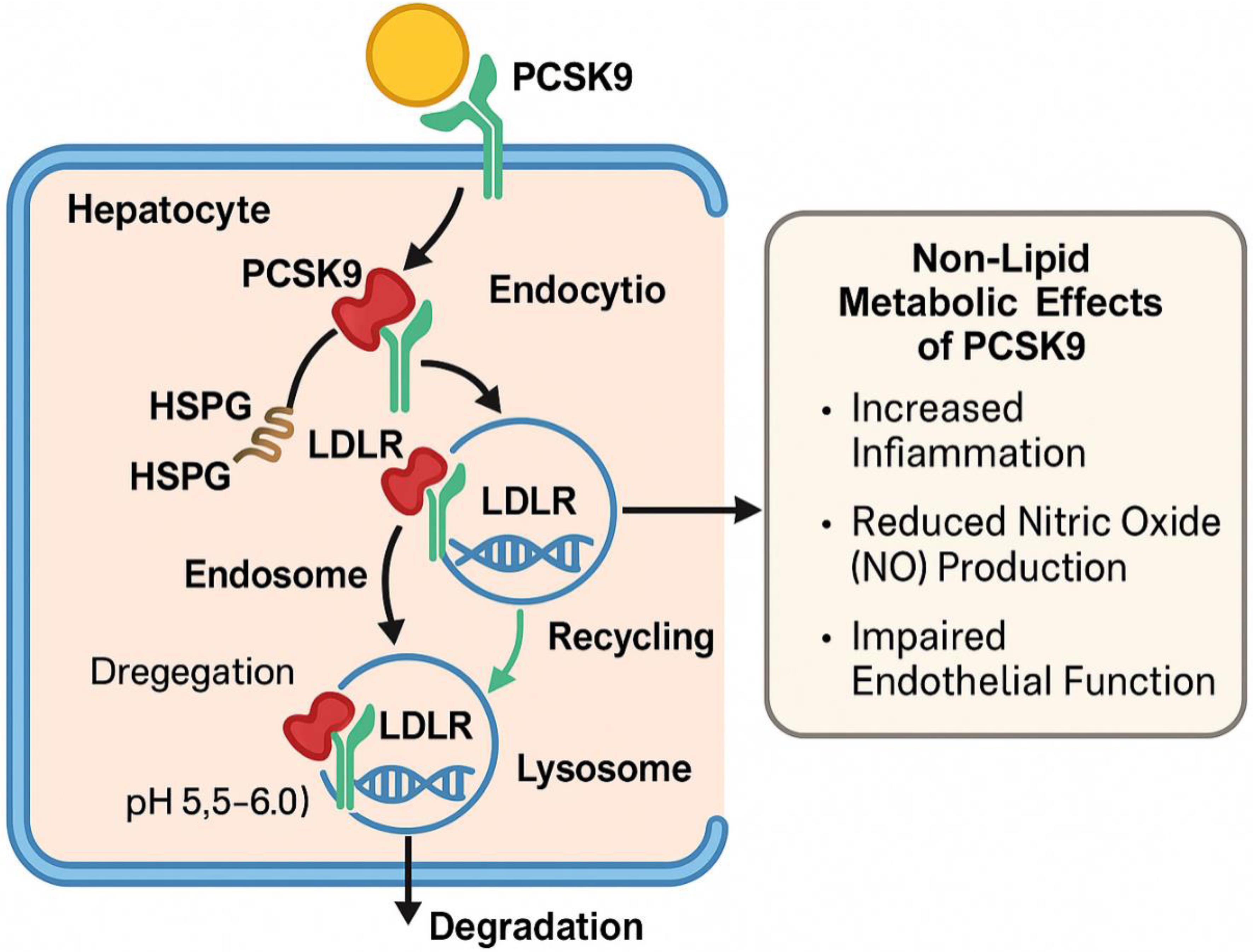
The LDLR, a type I transmembrane receptor, is commonly found on the plasma membranes of hepatocytes and features an extracellular domain located in the sinusoidal area. This receptor can bind extracellular ligands. To capture circulating LDL-C, the LDLR adopts an open conformation under physiological pH conditions. The clathrin-coated pits that internalize the LDLR/LDL-C complex then fuse with the endosomal compartment.23–26
Heparan sulfate proteoglycans that bind circulating PCSK9 deliver it to the LDLR. Receptor recycling in endosomes is enabled by the decreased pH, which causes LDLR/LDL-C dissociation and reorganization of LDLR from an active to inactive configuration. However, LDLR-mediated PCSK9 uptake impedes the conformational shift necessary for receptor recycling, directing the LDLR/PCSK9 complex toward lysosomal degradation.1 The reduced pH in endosomes prevents the conformational shift needed for LDLR recycling and amplifies the LDLR/PCSK9 interaction by a factor of 150. As a result, the LDLR/PCSK9 complex is targeted to lysosomes where it is degraded.27–29
This figure illustrates how PCSK9 binds to LDLR on hepatocytes, leading to internalization of the PCSK9-LDLR complex. Heparan sulfate proteoglycans (HSPGs) assist in mediating PCSK9 uptake. In the endosome, the acidic environment (pH 5.5–6.0) stabilizes the PCSK9-LDLR complex and inhibits receptor recycling. Instead, the complex is directed to lysosomal degradation, reducing surface LDLR and limiting low-density lipoprotein cholesterol (LDL-C) clearance. On the right, the non-lipid metabolic effects of PCSK9 are shown, including promotion of inflammation (e.g., via interleukin-6 and nuclear factor-kappa B signaling), reduced nitric oxide (NO) bioavailability, and endothelial dysfunction, which contribute to the progression of atherosclerotic cardiovascular disease.
Currently, two antibody-based PCSK9 inhibitors have reached the clinical stage, showing no significant adverse effects while effectively lowering cholesterol levels and reducing the risk of ASCVD occurrences, including stroke, myocardial infarction, and mortality.1 A vast excess (more than 100:1) of antibodies is introduced by subcutaneous injections of 75–150 mg of alirocumab or 140–420 mg of evolocumab, which bind all circulating PCSK9 within hours of administration and continue to capture newly secreted PCSK9 over the following days.30,31 ASCVD rates have decreased in tandem with the significant LDL-C reductions, often to levels below 25 mg/dL.22
PCSK9 and LDL-C regulation
Effectively lowering blood cholesterol levels reduces the risk of ASCVD, including CAD, which is the leading cause of mortality worldwide.1 Use of evolocumab in homozygous familial hypercholesterolemia was linked to a 57.6% decrease in LDL-C concentration at its lowest point. There is no clear explanation for this discrepancy; it could be that patients with homozygous familial hypercholesterolemia who were not genotyped had heterozygous familial hypercholesterolemia (HeFH) with only one mutated allele but a very severe LDL-C phenotype, or it could be that the patients included had biallelic mutations that moderately affected LDLR residual function. In patients with HeFH, our primary analysis showed that the addition of PCSK9 inhibitors led to an overall 56% reduction in LDL-C levels, a benefit that was consistent in the sensitivity analysis as well. However, only 42% of patients with HeFH achieved the 2019 individual LDL-C targets recommended by the European Atherosclerosis Society/European Society of Cardiology (<55 mg/dL in secondary prevention and <70 mg/dL in primary prevention).32
PCSK9 inhibitors reduced plasma LDL-C levels by nearly 60%, even in individuals already receiving the maximum dose of statin therapy.33 PCSK9 monoclonal antibodies significantly decreased LDL-C and reduced the relative risk of ASCVD occurrences by about 20% in placebo-controlled trials involving patients undergoing the most intensive lipid-lowering regimens they could tolerate.34 There are two dosages of alirocumab: 300 mg once a month, and 75 mg or 150 mg administered subcutaneously (SC) every two weeks. Although the higher dose can be started immediately, the lower dose is typically recommended as the starting dose. It is advised to evaluate the impact of this class on LDL-C at the trough level of the dosing interval, which is 14 days after the previous dose, to monitor its effects. LDL-C values decrease by 45% to 48% with the 75 mg dose and by roughly 60% with the 150 mg dose.35–38
Two dosing regimens for evolocumab are available, both of which reduce LDL-C by about 60%: 140 mg SC every two weeks, or 420 mg SC once a month.39 Evolocumab was found to lower the risk of coronary revascularization (odds ratio (OR) 0.77; 95% confidence interval (CI): 0.70–0.84; p < 0.01), stroke (OR 0.79; 95% CI: 0.66–0.94; p = 0.01), myocardial infarction (OR 0.72; 95% CI: 0.64–0.81; p < 0.01), and overall major adverse cardiac events (OR 0.85; 95% CI: 0.80–0.89; p < 0.01) (n = 42,637). The study also found that alirocumab decreased all-cause death (OR 0.60; 95% CI: 0.43–0.84; p < 0.01), myocardial infarction (OR 0.57; 95% CI: 0.38–0.86; p = 0.01), cardiovascular mortality (OR 0.35; 95% CI: 0.16–0.77; p = 0.01), and the overall incidence of major adverse cardiac events (OR 0.35; 95% CI: 0.16–0.77; p = 0.01).40
In patients with familial hypercholesterolemia who had not yet developed clinical ASCVD, adding alirocumab to high-intensity statin therapy led to a substantial reduction in coronary plaque load and plaque stabilization, as detected by coronary computed tomographic angiography over a 78-week period.41
Even without a statistically significant difference in high-sensitivity C-reactive protein, patients taking alirocumab experienced significantly greater reductions in lipoprotein (a), apolipoprotein (b), and triglycerides.42 This can be a useful tool for healthcare systems looking to incorporate PCSK9 inhibitors.43
PCSK9 inhibitors have also been demonstrated to enhance endothelial function and exhibit anti-inflammatory properties, which may further contribute to their beneficial effects on the cardiovascular system. It has been demonstrated that PCSK9 inhibitors lower inflammatory markers such as interleukin-6. Additionally, by boosting the synthesis of nitric oxide, a compound that dilates blood vessels and enhances blood flow, they can improve endothelial function.40
Efficacy of PCSK9 inhibitors in CAD prevention
After long-term treatment, evolocumab appears to have been both safe and effective, according to recent data.12 The observed therapeutic benefit of PCSK9 inhibitors in these trials occurred in the context of lowering LDL-C levels to previously unprecedented levels, indicating the need for more aggressive LDL-C targets.33 It was recently demonstrated that in patients with stable CVD using statins, evolocumab significantly lowers the risk of cardiovascular events by 15–20% (FOURIER study).20 In two distinct populations at high risk for CVD, a regimen of subcutaneous inclisiran injections on day 1, day 90, and then every six months decreased LDL-C levels by 49.9% to 52.2% at month 17 and decreased time-adjusted LDL-C levels between months 3 and 18 by 49.2% to 53.8% compared with placebo. The results for the percentage change in LDL-C levels at month 17 were consistent across subgroups. PCSK9 levels generally increased among patients who received a placebo, while PCSK9 levels decreased in almost all patients receiving inclisiran.44 A comparative summary of lipid-lowering therapies is presented in Table 1.6,20,21,45-52
Comparison of lipid-lowering therapies
| Therapy | LDL-C reduction (%) | Administration | Common adverse effects | Cardiovascular event risk reduction | Cost-effectiveness | Data source(s) |
|---|---|---|---|---|---|---|
| Statins | 30–50% | Daily oral | Myalgia, ↑ liver enzymes | 30–35% (primary/secondary prevention) | High (generics) | JUPITER,45 HOPE-36 |
| Ezetimibe | ∼18–25% | Daily oral | GI upset, ↑ liver enzymes | ∼6–8% (as statin add-on) | High (generic) | IMPROVE-IT46 |
| PCSK9 inhibitors | 50–60% | SubQ every 2–4 weeks | Injection-site reactions, flu-like symptoms | 15–20% (high-risk ASCVD) | Moderate–Low (expensive) | FOURIER,20 ODYSSEY Outcomes21 |
| Inclisiran | ∼50% | SubQ every 6 months | Injection-site reactions | Not yet fully reported | Moderate | ORION-10/1145 |
| Bempedoic acid | ∼15–25% | Daily oral | Gout, ↑ uric acid, mild GI upset | ∼13% (statin-intolerant CAD) | Moderate | CLEAR Outcomes47 |
| Niacin (vitamin B3) | ∼10–20% (↑ HDL-C) | Daily oral | Flushing, hepatotoxicity, hyperglycemia | No added benefit in modern trials | Low | AIM-HIGH,48 HPS2-THRIVE49 |
| Fibrates (e.g., fenofibrate) | Minimal (mainly ↓ TGs) | Daily oral | GI upset, ↑ liver enzymes, ↑ creatinine | Modest benefit in diabetic dyslipidemia | Moderate | FIELD,50 ACCORD51 |
| Icosapent ethyl | Neutral (↑ HDL, ↓ TGs) | Daily oral | GI upset, ↑ AFib risk (rare) | ∼25% (high TG + statin) | Moderate–High | REDUCE-IT52 |
Non-lipid metabolic effects of PCSK9
Besides its well-known involvement in lipid metabolism, there is evidence linking PCSK9 to thrombotic risk in both human and animal studies. Animal models found that PCSK9−/− mice had a relatively shorter thrombus duration following closure of the inferior vena cava and subsequent venous thrombosis, as well as lower leukocyte attachment and accumulation, neutrophil extracellular trap generation, and NETosis.53 Patients with antiphospholipid antibodies who were at high thrombotic risk had elevated PCSK9 levels. In patients with stable CAD, there was also a positive association between PCSK9 and fibrinogen levels; these findings indicate that PCSK9 and the coagulation system are positively correlated.54 Recent clinical research has demonstrated that patients with heart failure, particularly heart failure with reduced ejection fraction, have substantially higher circulating levels of PCSK9. Furthermore, in patients experiencing an exacerbation of heart failure, elevated PCSK9 levels are a negative prognostic indicator. However, there are also reports of loss-of-function (LOF) mutations involving disruption of local PCSK9 function in the heart, which leads to lipid buildup in cardiomyocytes.55
Safety and adverse effects
Patients with peripheral artery disease face an elevated risk of cardiovascular events; however, inhibition of PCSK9 with evolocumab markedly reduces this risk, showing substantial overall risk reduction.56 Each PCSK9 inhibitor has an excellent safety profile, with nasopharyngitis and mild injection-site reactions being the most frequently occurring adverse effects. Importantly, at very low LDL-C levels, there is no increase in myalgias and, crucially, no negative effect on neurocognitive function.22 According to studies, lowering LDL-C levels below 50 mg/dL while taking statins is safe and may provide additional cardiovascular benefits. However, the multi-beneficial profile of statins may influence the safety outcomes and cardiovascular effects. The results of the IMPROVE-IT trial, which evaluated the effects of intensive treatment with ezetimibe in addition to statins, were encouraging. Studies using PCSK9 inhibitors, a novel class of monoclonal antibodies that block PCSK9 binding to the LDLR and prevent its degradation, provide crucial information regarding the safety of low and very low LDL-C levels by lowering plasma LDL-C levels. Two medications in this class used to treat hyperlipidemia are evolocumab and alirocumab. The FOURIER trial collected clinically significant data by randomly assigning more than 27,000 patients with overt CVD and LDL-C levels of 70 mg/dL to either evolocumab or placebo for 168 weeks of follow-up. In the evolocumab group, LDL-C values were lowered to less than 40 mg/dL in 67% of patients and to less than 25 mg/dL in 42% of patients.57
Cost effectiveness
Although there is a lot of excitement around these new medications, questions have been raised about their high price and potential benefits to the healthcare system.58 These inhibitors are not cost-effective for the majority of people due to their exorbitant cost, which is $15,000 in the USA and $7,000 in other industrialized nations. It might be more economical to use these inhibitors in patients with familial hypercholesterolemia and statin intolerance instead.59 In the UK, PCSK9 inhibitors can cost up to £4,400 annually, which exceeds the National Health Service’s cost-effectiveness threshold of £20,000 to £30,000 per quality-adjusted life year. Accordingly, they are a “double-edged sword” in treating patients with dyslipidemia. Pharmaceutical companies charge exorbitant prices for products with limited effectiveness. With annual treatment costs of about £4,400, the National Health Service concluded that neither of the PCSK9 inhibitors was cost-effective. To be cost-effective, PCSK9 inhibitors must be discounted by more than 37%. Patients with a high risk of acute cardiovascular events, existing CVD, and LDL-C levels greater than 135 mg/dL are not eligible for prescriptions. By cutting costs and limiting prescriptions to individuals who are at risk, incremental cost-effectiveness ratios can be significantly improved.60
Expanding the scope: PCSK9 inhibition beyond dyslipidemia
It has been proposed that PCSK9 plays a role in the pathophysiology of dyslipidemia in nephrotic kidney disease. Nephrotic renal disease is associated with higher plasma PCSK9 levels. According to research by Haas et al.,61 plasma PCSK9 levels dramatically dropped in nephrotic patients when their hypercholesterolemia improved with remission. Treatment with statins had no effect on this outcome.61–63 Human studies also revealed that people with PCSK9 LOF mutations exhibited a decreased risk of septic shock accompanied by less severe organ damage.64,65 PCSK9 is involved in apoptosis, differentiation, and cholesterol metabolism in cultured neuronal cells. In a rat model of reperfusion injury/cardiac ischemia, pre-treatment with a PCSK9 inhibitory peptide demonstrated protective effects against neuronal damage.66,67 People with PCSK9 LOF mutations do not appear to have any neurological impairments.68
A recent study discovered that PCSK9 modulates the expression of multiple cell surface receptors on human pancreatic beta cells.69 While the impact of pancreatic islet beta cell-derived PCSK9 on glucose-stimulated insulin release and diabetes risk remains unclear, it is known that PCSK9 is secreted and causes autocrine degradation of LDLR.70 Nevertheless, treatment with evolocumab for 2.2 years did not raise the incidence of diabetes or glycemia in either group, indicating that PCSK9 inhibitors and statins may have distinct effects on the pancreas’s ability to absorb harmful LDL-C.71 PCSK9 has also been linked in various ways to cancer biology. Notably, variations in PCSK9 LOF and gain-of-function mutations have been associated with better and worse breast cancer survival rates, respectively.72 In several tumor mouse models, PCSK9 inhibitory monoclonal antibodies improved survival, an outcome that was found to work synergistically with immune checkpoint inhibition using anti-PD1 monoclonal antibodies.73
Future directions and ongoing research
New therapeutic indications for PCSK9 inhibitors are being explored in ongoing trials, and their cost-effectiveness is still being evaluated. A new class of hypolipidemic medications called PCSK9 inhibitors is now recommended for high-risk patients for secondary ASCVD prophylaxis to lower residual risk.12 The development of the PCSK9 inhibitor field is expected to extend far beyond the traditional paradigm of lowering LDL-C. Current trials are investigating various improvements and novel therapeutic applications, paving the way for additional advances in the treatment of cardiovascular and broader metabolic diseases.
Limitations
This review is based on a collection of recent literature and clinical trial evidence and is potentially influenced by publication bias. While the included studies provide helpful information, discrepancies in study design, populations, and outcomes may affect the generalizability of the conclusions. Furthermore, long-term real-world data on the non-lipid effects of PCSK9 inhibitors remain limited and require further investigation.
9. Conclusions
Patients with multiple CAD lesions who have not met lipid-lowering targets have shown significant improvement in LDL-C concentrations thanks to PCSK9 inhibitors. This enables doctors to better control their patients’ cholesterol levels, thereby lowering their risk of CVD. Patients treated with PCSK9 inhibitors, compared to conventional therapy, showed greater improvement in angina flare-up, angina stability, physical limitation, and treatment satisfaction scores. One out of every three cardiac patients with HeFH may benefit from PCSK9 inhibitor therapy.
Declarations
Acknowledgement
None.
Funding
None.
Conflict of interest
The authors declare no competing interests.
Authors’ contributions
Literature review (FP, FD), conceptualization, data extraction, writing – original draft preparation (FP), methodology (FD), formal analysis, investigation (MA, FV), and literature collection (FV). All authors have read and approved the final version of the manuscript for publication.

 Author information
Author information