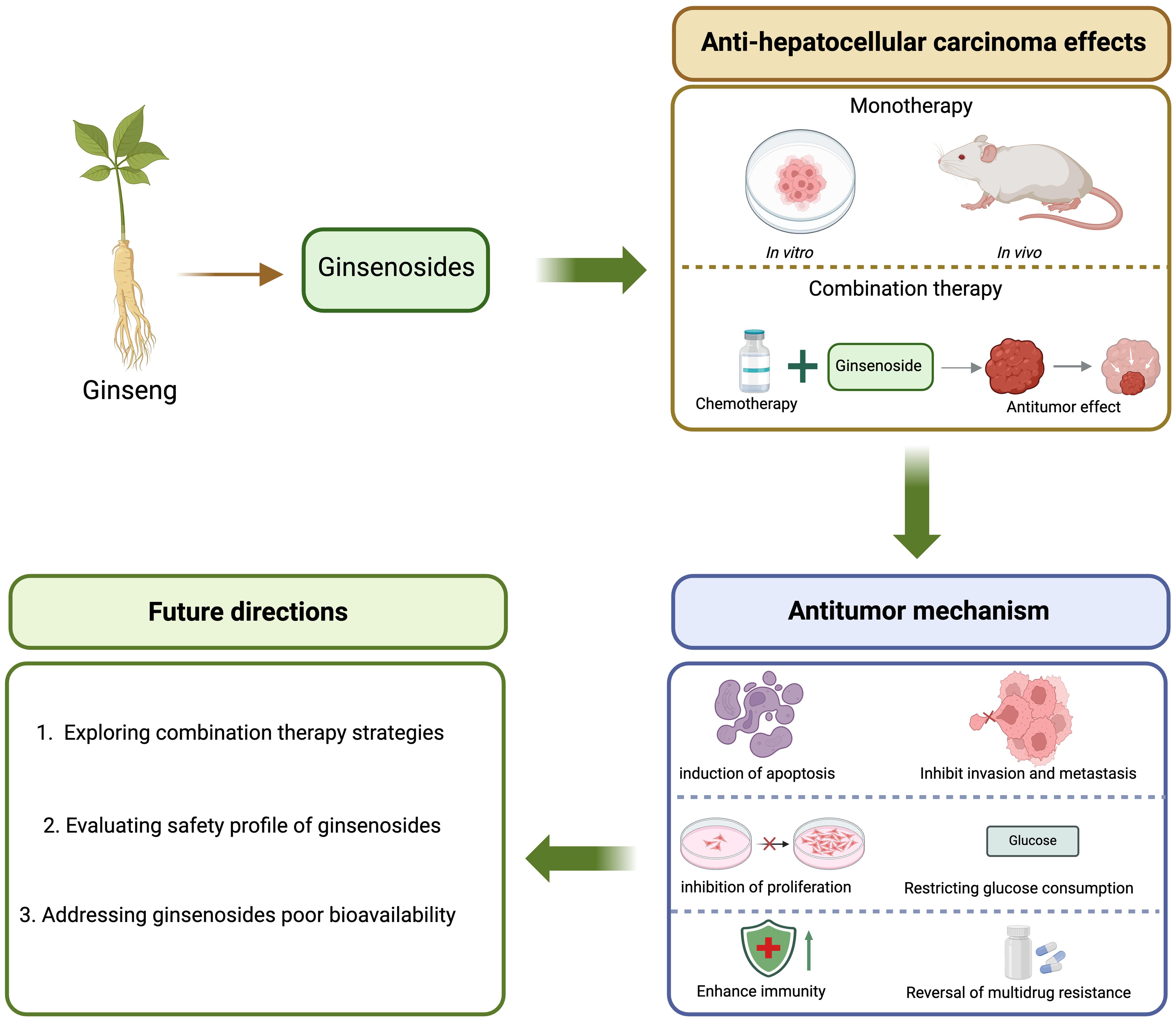
Introduction
Primary liver cancer is one of the most common malignant tumors worldwide, with approximately 865,269 new cases and 757,948 deaths reported globally in 2022, making it the third leading cause of cancer-related mortality.1–4 Hepatocellular carcinoma (HCC) represents the predominant pathological type, accounting for 75–85% of primary liver cancer cases.5,6 Major risk factors include chronic hepatitis B/C virus infection, cirrhosis, and metabolic diseases.7 Due to its asymptomatic early stages, most patients are diagnosed at advanced stages.7 Current therapeutic approaches include surgical resection, liver transplantation, transarterial chemoembolization (TACE), radiofrequency ablation, and targeted therapy, yet challenges such as tumor recurrence, drug resistance, and adverse effects persist.8 Although liver transplantation remains a curative option, its application is limited by donor shortage and post-transplant recurrence.9 The overall prognosis of HCC remains poor, with a five-year survival rate of only ∼10%,10 and palliative care is often administered for advanced-stage patients.11
Traditional Chinese medicine (TCM) serves as an important component of comprehensive treatment, demonstrating the potential to prolong patient survival,12 improve postoperative survival rates, and enhance quality of life.13,14 For instance, herbal formulations such as Fuzheng Jiedu Xiaoji formulation have shown clinically confirmed efficacy.15 Integrated Chinese-Western therapy (e.g., TCM combined with radiofrequency ablation) has been reported to improve short-term therapeutic outcomes and quality of life.16 Network pharmacology studies reveal that TCM exerts multi-target inhibitory effects on HCC progression, including modulation of key signaling pathways.17 Additionally, TCM demonstrates potential in delaying disease progression and mitigating adverse effects associated with radiotherapy and chemotherapy.18
Recent studies have revealed that ginsenosides, the primary bioactive constituents of Panax ginseng, exhibit multi-faceted potential in cancer therapy. In 1983, Japanese researchers first isolated ginsenoside Rg3 (GRg3) from Korean red ginseng. As a tetracyclic triterpenoid dammarane-type rare ginsenoside, GRg3 is present in low quantities in fresh ginseng, American ginseng, and Panax notoginseng roots/rhizomes, but accumulates at higher levels in aged ginseng, ginseng stems/leaves, processed red ginseng, and black ginseng.19 Structurally classified as a protopanaxadiol-type saponin, GRg3 has a molecular weight of 784 Da. It can be derived from the thermal or enzymatic degradation of natural ginsenosides Rb1, Rb2, and Rd.19 The spatial configuration at the C20 position generates two enantiomers: 20(S)-Rg3 and 20(R)-Rg3.20 The tertiary structural differences between these isomers result in superior anti-tumor activity of 20(S)-Rg3 compared to its R-form. Specifically, 20(S)-Rg3 demonstrates significantly stronger inhibition of HepG2 cell proliferation and apoptosis induction.21 The alkene chain in the aglycone moiety of 20(S)-Rg3 forms tight hydrophobic packing near C20, enhancing hydrogen bonding with Ca2+/Na+ ion channels and ligand-gated ion channels, thereby strengthening interactions with receptor domains.22
Current evidence indicates that various ginsenoside subtypes exert anti-tumor effects through multiple mechanisms, including induction of apoptosis, suppression of proliferation and metastasis, modulation of the tumor microenvironment, and reversal of chemotherapy resistance.
This review aims to systematically summarize the multi-targeted anti-HCC mechanisms of ginsenosides, discuss their synergistic potential with conventional therapies, and highlight future directions for clinical translation (Fig. 1).
Inhibition of HCC cell proliferation
The pathogenesis of HCC is closely associated with an imbalance between cell proliferation and apoptosis, where excessive proliferation over apoptosis leads to malignant tumor growth. Ginsenoside compound K suppresses HepG2 cell proliferation by inducing ferroptosis.23,24 Its acetylated derivative, compound K-3, further enhances the antitumor effect by arresting the cell cycle at the G2/M phase and inducing apoptosis.24
Additionally, ginsenoside Rk1 exerts anti-proliferative effects in HCC models by inducing autophagy-dependent apoptosis.25 Rg3 effectively inhibits the growth of human HCC cell lines through dual mechanisms: suppressing cancer cell proliferation and promoting apoptosis. This pro-apoptotic effect may occur via the intrinsic mitochondrial-mediated caspase-dependent pathway.26
Notably, Rg3 inhibits HCC cell proliferation and induces apoptosis in a concentration- and time-dependent manner in vitro. Studies demonstrate that Rg3, either alone or in combination with cyclophosphamide, suppresses tumor growth in vivo and prolongs survival in mouse models. This effect is mediated through the intrinsic apoptotic pathway, involving altered expression of B-cell lymphoma 2 (Bcl-2) family proteins.27
Inhibition of HCC cell invasion and metastasis
Tumor invasion and metastasis constitute a complex, multi-step cascade process regulated by distinct genetic mechanisms. Both ginsenosides Rh1 and Rb1 demonstrate concentration- and time-dependent inhibition of HepG2 cell invasion and metastasis.28,29 Rd-treated HepG2 cells exhibit significantly suppressed metastatic potential, primarily through inhibition of matrix metalloproteinase (MMP) activation and modulation of the MAPK signaling pathway involved in tumor cell migration.30
Ginsenoside Rh2 effectively attenuates the migratory capacity of HepG2 cells by activating glycogen synthase kinase-3 beta, degrading β-catenin, and downregulating metastasis-related proteins such as MMP3, thereby reducing tumor invasion and migration.31 Furthermore, compound K, a metabolite of ginsenosides, has also demonstrated anti-invasive and anti-metastatic properties, which may be attributed to the translocation of nuclear factor-κB p65 and the reduction of MMP-2 and MMP-9.32 Additionally, in the diethylnitrosamine-induced HCC mouse model, NpRg3 (Fe@Fe3O4 conjugated with GRg3) eliminates HCC lung metastasis, possibly by remodeling gut microbiota and metabolism.33
Induction of apoptosis in HCC cells
One of the key factors contributing to the rapid growth of HCC is the reduced apoptosis of tumor cells. Consequently, artificial induction of apoptosis in HCC cells has become a research focus in anticancer therapy. Ginsenoside Rh2 has been demonstrated to effectively induce apoptosis in human SK-HEP-1 HCC cells. The pro-apoptotic mechanisms of ginsenosides against HCC primarily involve the following molecular pathways:
Ginsenosides can regulate apoptosis-related proteins. For instance, GRg3 and G protein-coupled receptor kinase 1 (GRk1) promote apoptosis in HCC cells by modulating the expression of apoptosis-associated proteins. Studies indicate that GRg3 and GRk1 may induce apoptosis through downregulation of the anti-apoptotic protein Bcl-2 and upregulation of pro-apoptotic proteins such as caspase-3.34,35 Additionally, research demonstrates that ginsenoside compound K can influence the expression of other apoptosis-related proteins, including caspase-8, Fas, and FasL.36
Rh2 induces rapid and significant translocation of pro-apoptotic proteins Bax and Bak, subsequently triggering mitochondrial cytochrome C release and caspase activation.37 Experimental studies revealed that small interfering RNA-based caspase-8 gene inactivation effectively delays Rh2-induced caspase-9 activation and apoptosis. These findings suggest that Rh2 employs multiple pro-apoptotic pathways to execute cancer cell death.37 Investigations on the effects of Korean red ginseng and its major components Rg3 and Rh2 on apoptosis in human Hep3B HCC cells showed decreased cell viability and mitochondrial membrane potential, along with increased lactate dehydrogenase release, confirming the significant cytotoxic and pro-apoptotic effects of Korean red ginseng, Rg3, and Rh2 on Hep3B cells.38
Ginsenosides can inhibit the PI3K/AKT signaling pathway. For example, GRk3 enhances apoptosis in HCC cells by suppressing the PI3K/AKT pathway. As this pathway serves as a crucial regulator of cell survival and proliferation, its inhibition by GRk3 leads to enhanced pro-apoptotic effects.39
Ginsenosides can modulate tumor metabolism. Specifically, ginsenoside Rk1 inhibits HCC proliferation by regulating glutamine metabolism. This mechanism involves suppression of the ERK/c-Myc signaling pathway, downregulation of glutaminase glutaminase 1 expression, reduction in glutathione production, and concurrent increase in reactive oxygen species accumulation, thereby promoting apoptosis both in vitro and in vivo.35
In summary, ginsenosides induce apoptosis in HCC cells through multiple pathways, including the regulation of apoptosis-related proteins (e.g., Bcl-2, caspases), inhibition of the PI3K/AKT pathway, modulation of tumor metabolism, and oxidative stress. These mechanisms collectively contribute to their anti-HCC efficacy.
Restricting glucose consumption
Due to impaired or lost glucagon inactivation in the liver of HCC patients, hepatogenous diabetes often develops, making blood glucose reduction an essential component of HCC treatment. Ginsenoside Rh4 inhibits inflammation-related HCC progression by targeting the HDAC4/IL-6/STAT3 signaling pathway. This mechanism involves downregulation of key glycolytic enzymes glucose transporter 1 and lactate dehydrogenase A, leading to reduced glucose consumption and lactic acid production, thereby suppressing glycolysis and HCC growth.40 Similarly, GRg3, in combination with sorafenib, inhibits HCC progression by suppressing HK2-mediated glycolysis, leading to reduced glucose consumption and lactate production.41
Reversal of multidrug resistance in HCC cells
Multidrug resistance refers to a phenomenon where tumor cells exposed to a particular chemotherapeutic agent develop resistance not only to that specific drug but also to other structurally unrelated compounds with different mechanisms of action. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) represents a promising anticancer agent due to its selective cytotoxicity against tumor cells. However, prolonged use often leads to drug resistance.
Studies have demonstrated that standardized red ginseng extract (RGE) and 20(S)-Rg3 can sensitize HCC cell lines to TRAIL-induced cell death.42 Experimental evidence confirms that RGE-associated upregulation of the transcription factor CCAAT/enhancer-binding protein homologous protein plays a crucial role in sensitizing TRAIL-mediated apoptosis. Furthermore, Rg3 has been shown to upregulate the expression of CCAAT/enhancer-binding protein homologous protein-mediated death receptor-5 at the transcriptional level, thereby enhancing TRAIL-induced apoptosis in various HCC cell lines. These findings suggest that both RGE and Rg3 may serve as effective chemosensitizers when combined with TRAIL therapy to reverse multidrug resistance in HCC cells.43,44
Enhancement of host immune function
The immune function of the organism is closely associated with tumor development and progression. Immunosuppression or compromised immune function leads to increased tumor incidence, while progressive tumor growth conversely exacerbates immune suppression, forming a reciprocal causal relationship. Consequently, immune enhancement has become an indispensable component of antitumor therapy. GRg3 has been demonstrated to inhibit the growth of H22 hepatoma cell xenografts in vivo, at least partially through a stereospecific mechanism that enhances host cellular immunity (Table 1).23-41,43,45-47,48
Structure and anti-hepatocellular carcinoma mechanism of ginsenosides
| Name | Structure | Mechanism | References |
|---|---|---|---|
| Ginsenoside Rg3 | 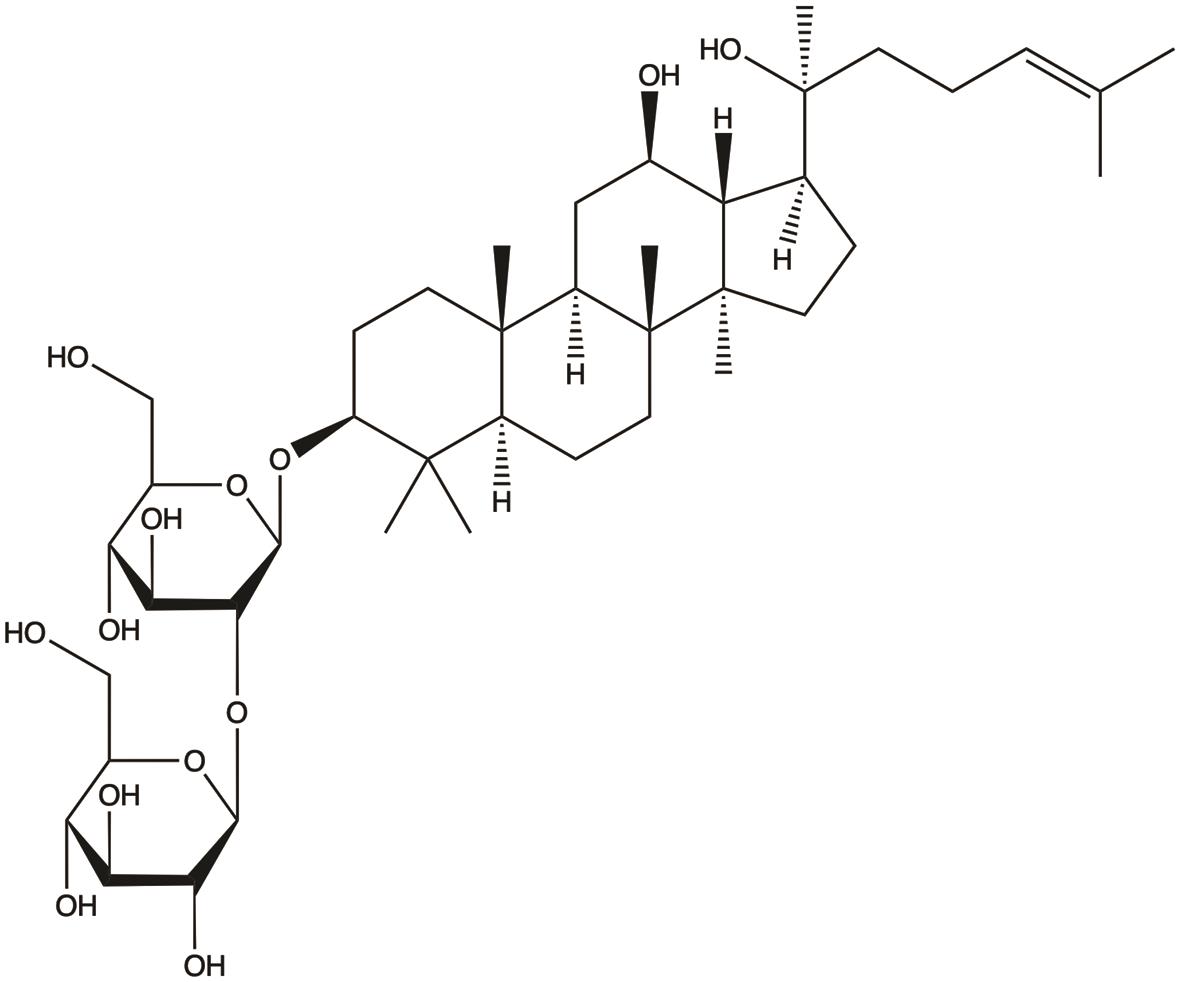 | 1. Inhibits proliferation; 2. Induces apoptosis; 3. Inhibits invasion and metastasis; 4. Suppresses glycolysis; 5. Enhances chemotherapy sensitivity; 6. Enhances host immunity; 7. Reversal of multidrug resistance; 8. Anti-angiogenesis | 26,27,33,34,41,43,45,47,48 |
| Ginsenoside Rh2 | 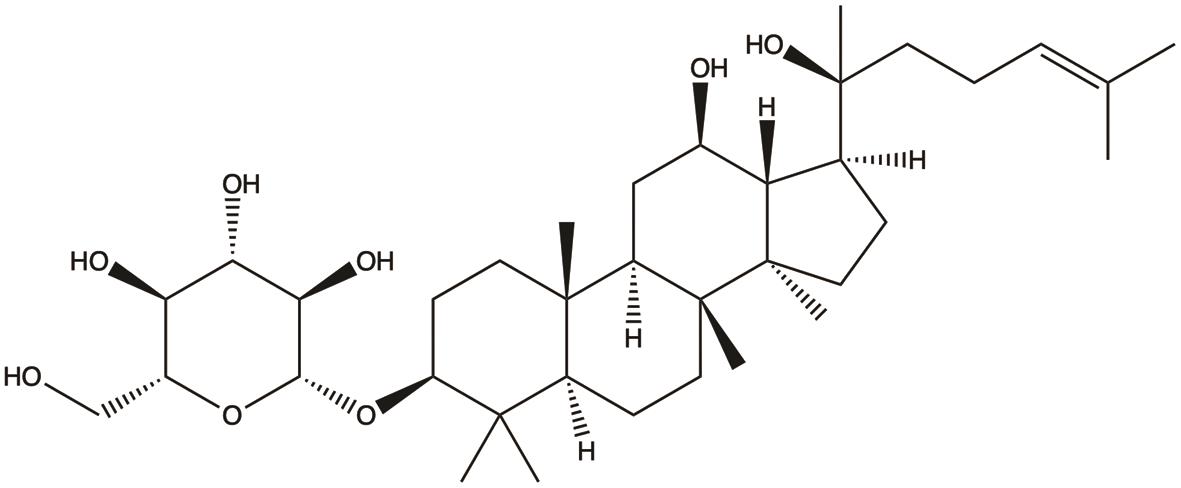 | 1. Inhibits proliferation; 2. Induces apoptosis; 3. Inhibits migration | 31,37,38 |
| Ginsenoside compound K | 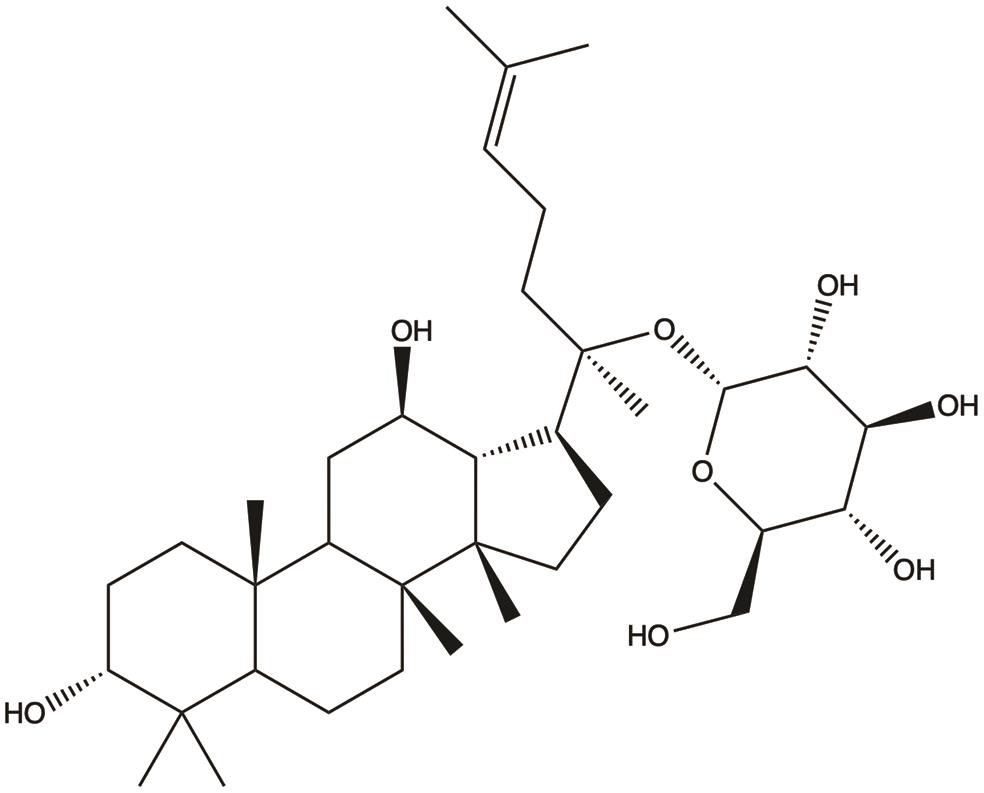 | 1. Induces ferroptosis; 2. Inhibits proliferation; 3. Suppresses metastasis; 4. Induces apoptosis | 23,24,32,36 |
| Ginsenoside Rk1 | 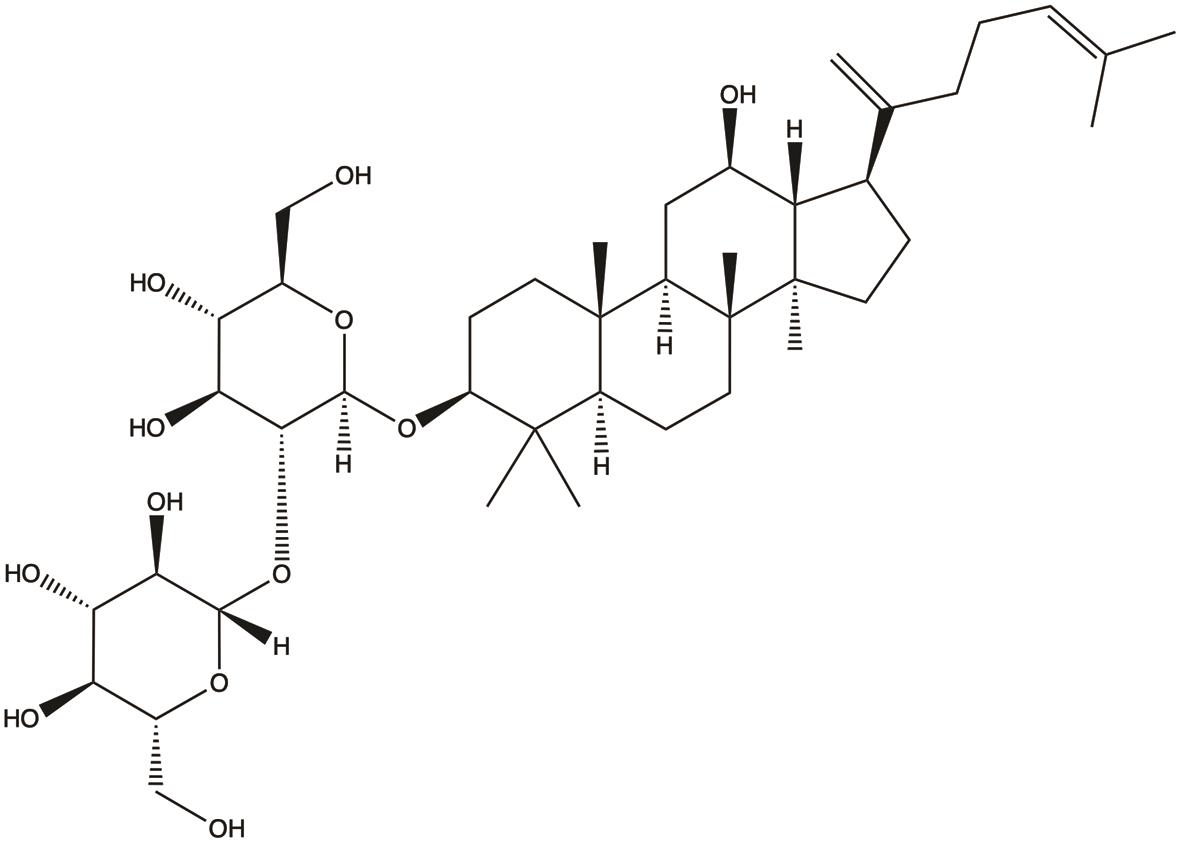 | 1. Inhibits proliferation; 2. Induces apoptosis | 25,35 |
| Ginsenoside Rh1 | 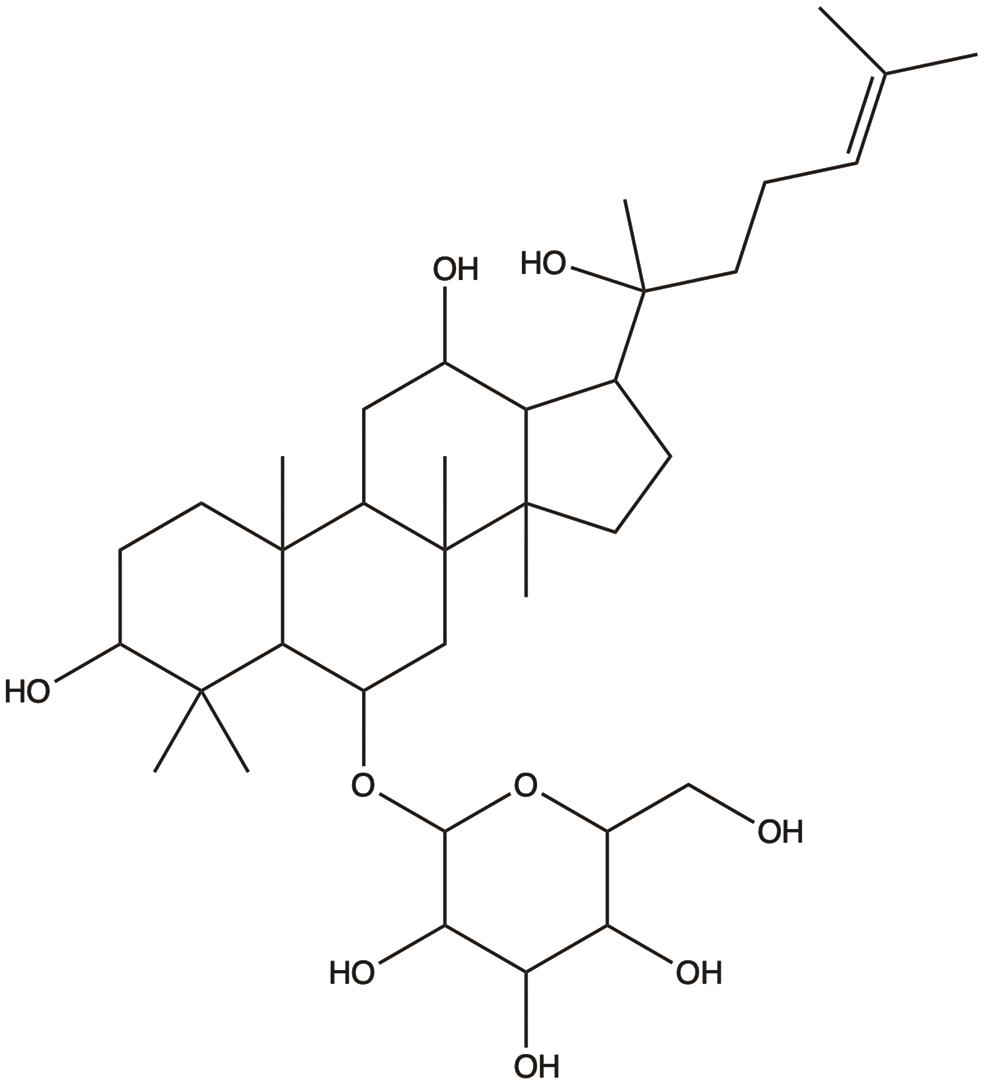 | 1. Inhibits invasion and metastasis | 29 |
| Ginsenoside Rd | 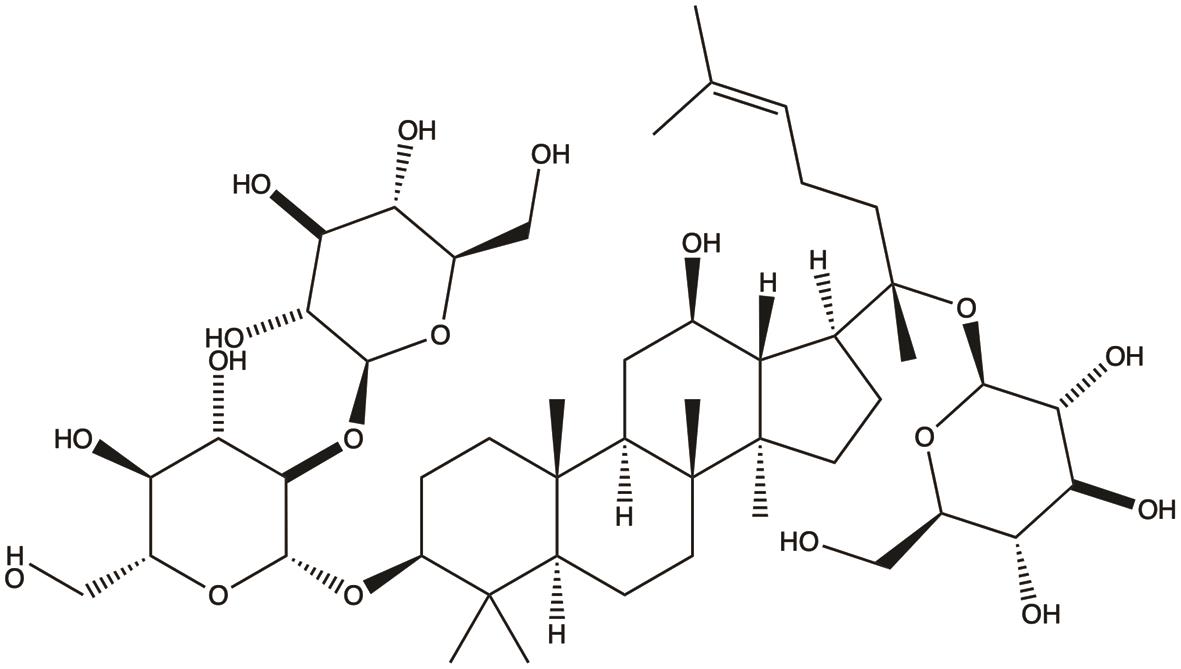 | 1. Inhibits invasion and metastasis | 30 |
| Ginsenoside Rb1 | 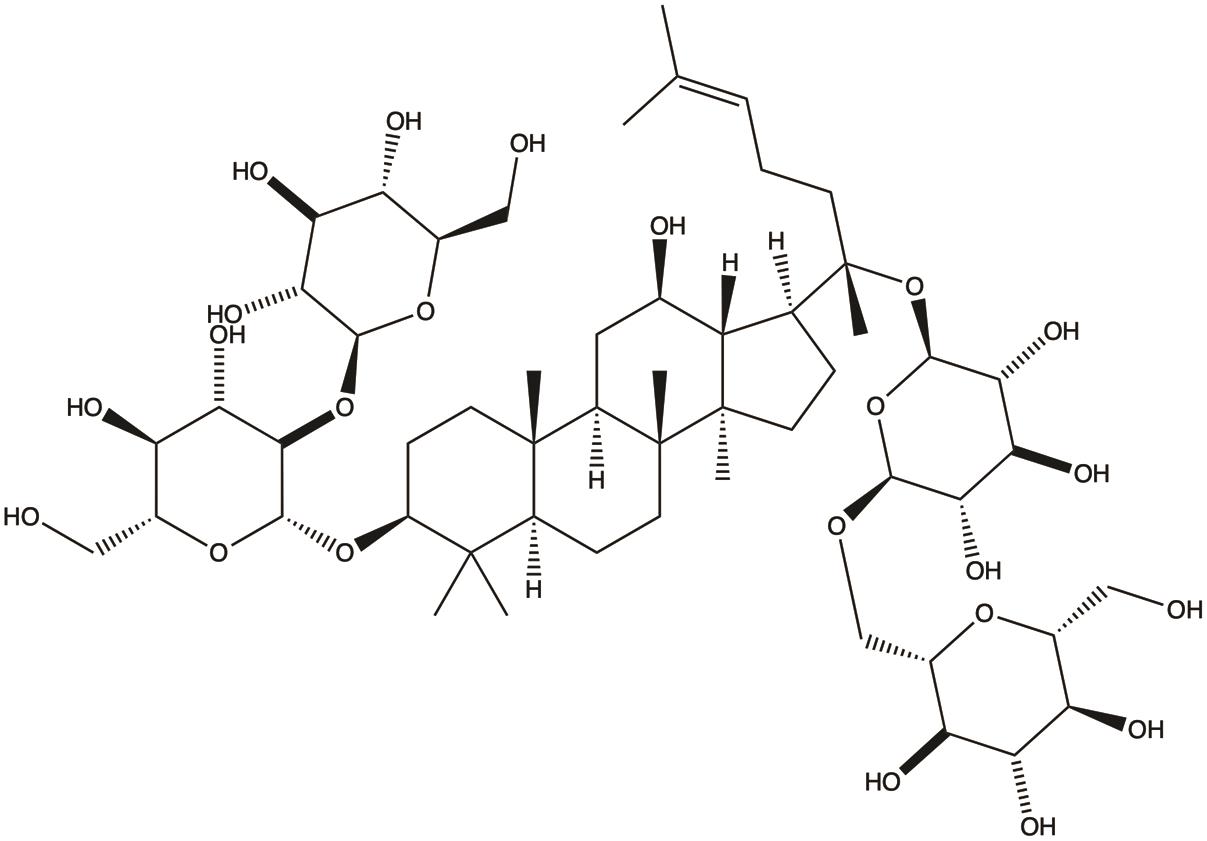 | 1. Inhibits invasion and metastasis; | 28 |
| Ginsenoside Rk3 | 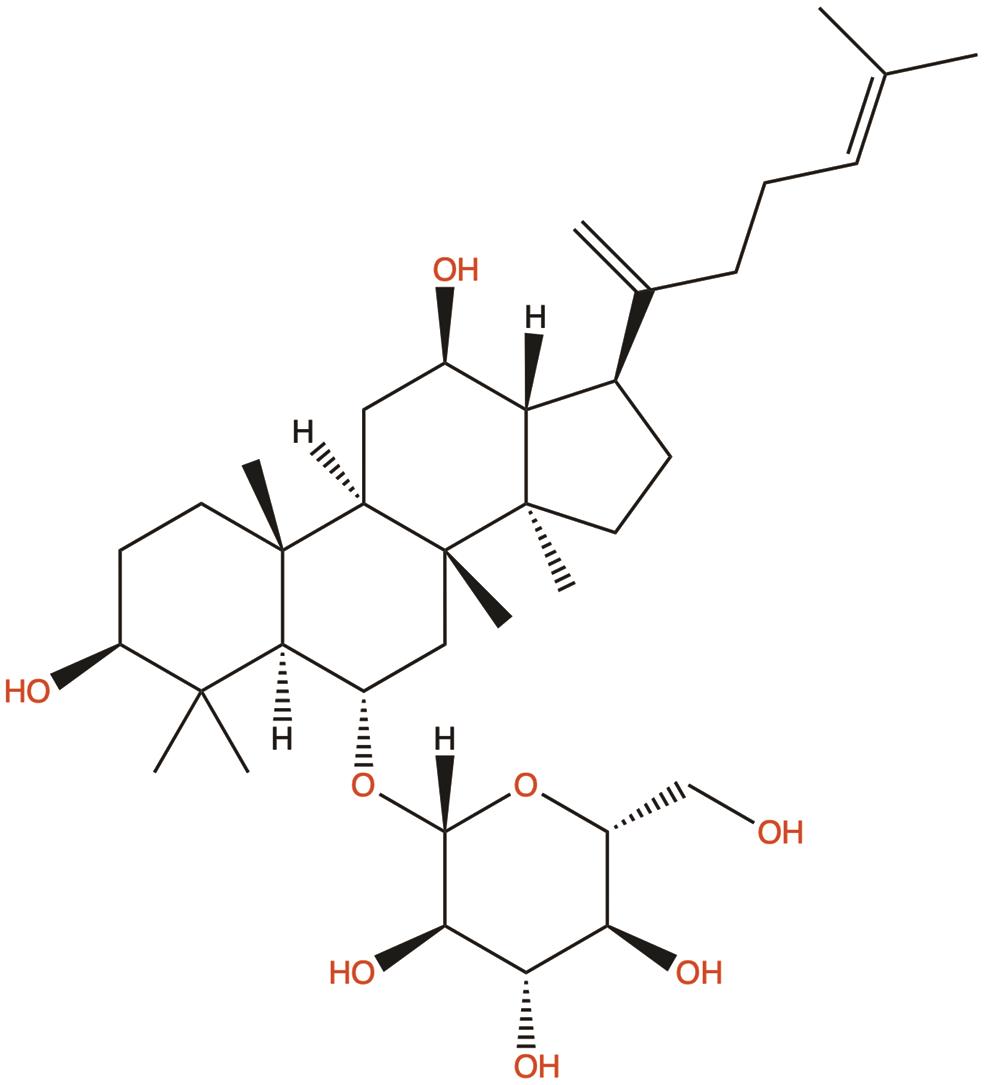 | 1. Induces apoptosis; 2. Induces autophagy | 39 |
| Ginsenoside Rh4 | 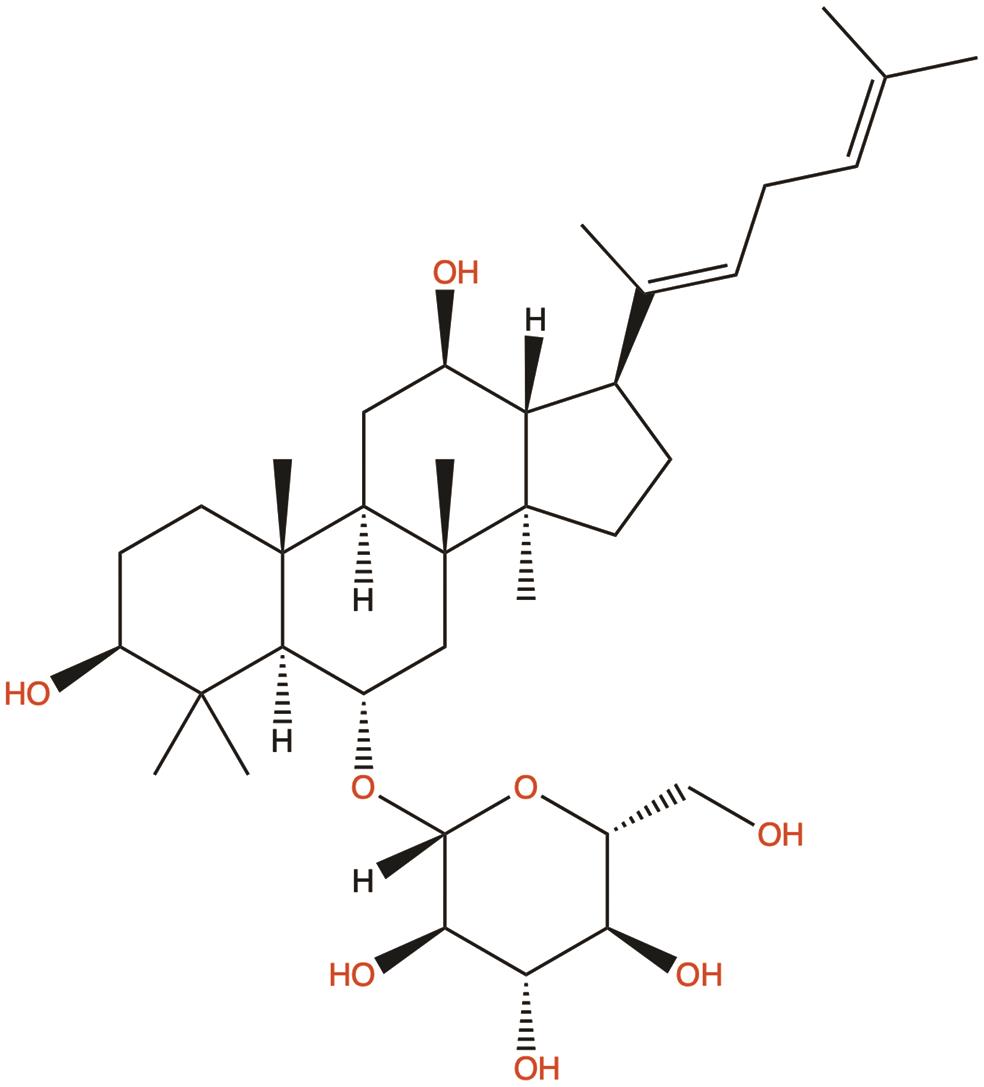 | 1. Suppresses glycolysis | 40 |
Synergistic effects in combination with other therapeutic approaches
Currently, monotherapy demonstrates limited efficacy in HCC treatment, making the combination of different therapeutic strategies to suppress tumor growth through multiple pathways a critical research direction.
Rg3 has been shown to inhibit autophagy and enhance the sensitivity of HCC cell lines to doxorubicin in vitro.47 Rh2, when combined with betulinic acid, synergistically induces apoptosis in human HCC HepG2 cells, suggesting that this combination could serve as a novel strategy to improve the efficacy of anticancer therapies.49 In a murine H22 HCC model, Shen-Ling-Bai-Zhu Powder—containing ginsenosides Rb1 and Rg1—potentiated the antitumor effect of cyclophosphamide. The combination synergistically suppressed tumor growth by inhibiting the Akt/NF-κB pathway, downregulating anti-apoptotic proteins (Bcl-2, Bcl-xL, Survivin), and enhancing pro-apoptotic caspase-3/9 activation. This demonstrates ginsenosides’ role in a multi-component formula to promote apoptosis and improve chemotherapy efficacy in HCC.50
In addition, an optimized QHF formula containing GRg3, cinobufotalin, panax notoginseng saponins, and lentinan was developed using a uniform design. In HCC mice, the QHF formula demonstrated superior antitumor efficacy (55.91% tumor inhibition, 38.13% prolonged survival) compared to any single agent. When combined with cisplatin (DDP), QHF synergistically enhanced tumor growth inhibition (82.54%) and survival (87.02%), while significantly alleviating DDP-induced toxicities such as leukopenia and immune organ atrophy. This highlights GRg3’s role within a multi-component formula to potentiate chemotherapy and reduce adverse effects in HCC treatment (Table 2).34,41–43,47,49–51
Summary of ginsenoside-based combination therapies for HCC
| Combination therapy regimen | Target (cell line/animal model) | Synergistic effects | References |
|---|---|---|---|
| Ginsenoside Rg3 + Cyclophosphamide | H22 HCC mouse model | Inhibits tumor growth and prolongs survival in mice | 34 |
| Ginsenoside Rg3 + Sorafenib | HCC cell lines / HCC mouse models | Inhibits HCC progression and reduces glucose consumption as well as lactate production | 41 |
| Ginsenoside Rg3 + Doxorubicin | HCC cell lines (in vitro) | Enhances the sensitivity of HCC cells to doxorubicin | 47 |
| Ginsenoside Rg3 (in QHF formula) + Cisplatin (DDP) | HCC mouse models | Improved tumor inhibition rate (82.54%), prolonged survival (87.02%), and alleviated DDP-induced toxicities (e.g., leukopenia, immune organ atrophy) | 51 |
| Ginsenoside Rh2 + Betulinic Acid | HepG2 HCC cell line (in vitro) | Synergistically induces apoptosis in HCC cells | 49 |
| Ginsenosides Rb1/Rg1 (in Shen-Ling-Bai-Zhu Powder) + Cyclophosphamide | H22 HCC mouse model | Inhibits tumor growth and promotes apoptosis of HCC cells | 50 |
| Standardized red ginseng extract (RGE) / 20(S)-Rg3 + TRAIL | HCC cell lines (in vitro) | Reverses TRAIL resistance and enhances TRAIL-induced apoptosis in HCC cells | 42,43 |
Key challenges in clinical translation
The compelling preclinical data summarized in this review firmly establish ginsenosides as promising anti-HCC agents. Their multi-targeted actions—inducing apoptosis, inhibiting metastasis, reversing multidrug resistance, and modulating immunity—align perfectly with the complex pathophysiology of HCC. This polypharmacological profile is particularly advantageous for treating a heterogeneous and adaptive cancer like HCC.
Building upon the promising preclinical evidence, the clinical efficacy of ginsenosides, particularly Rg3, has been preliminarily validated in a prospective, randomized controlled trial. A study by Zhou et al.52 demonstrated that in patients with advanced HCC (BCLC stage C), the combination of TACE and GRg3 significantly prolonged median overall survival compared to TACE alone (13.2 months vs. 10.1 months; HR = 0.63, P = 0.002), alongside an improved disease control rate and a reduction in several TACE-related adverse events. This landmark study provides a crucial clinical foundation for the application of ginsenosides in HCC management.
Despite these encouraging clinical findings, several significant challenges remain for the broader clinical translation of ginsenosides.
A significant hurdle for clinical translation is the inherently poor oral bioavailability of many ginsenosides.53 This is due to their large molecular size, low permeability, and extensive metabolism by gut microbiota. Efforts to overcome this include the development of novel drug delivery systems, such as nanoparticles, liposomes, and micelles, which can enhance solubility, protect against degradation, and promote targeted delivery to tumor tissue.
While some ginsenoside preparations (e.g., Yangzheng Xiaoji Capsule) are approved as adjuvants for cancer therapy in China, large-scale, randomized, placebo-controlled clinical trials specifically focused on HCC outcomes are still limited. Most existing human data come from retrospective studies or small-scale trials, which, while encouraging, are not sufficient to establish ginsenosides as standard care.
Future directions and strategies in translational research
Given their established role as chemosensitizers and radiosensitizers, a pragmatic clinical pathway is to develop ginsenosides as adjuvants to existing therapies. Combining ginsenosides with sorafenib or lenvatinib may enhance efficacy and overcome resistance. Furthermore, their integration with immune checkpoint inhibitors holds significant promise. Ginsenosides, through potent anti-angiogenic effects via vascular endothelial growth factor downregulation,48 can help normalize the immunosuppressive tumor microenvironment, thereby potentially augmenting T-cell infiltration and response to immunotherapy.54
Furthermore, a comprehensive safety profile and toxicity assessment of ginsenosides must be established as a priority for clinical translation. Although most preclinical studies have reported a favorable safety profile with no significant toxicity, contradictory reports of potential hepatotoxicity underscore the necessity for rigorous, systematic evaluation.55 Future investigations should therefore focus on elucidating the long-term safety, potential drug-drug interactions, and dose-limiting toxicities of ginsenosides, particularly in combination regimens. Defining the therapeutic window will be crucial for optimizing dosing schedules and mitigating adverse effects, ensuring that the promising efficacy of ginsenoside-based therapies can be safely realized in a broader patient population.
Moreover, overcoming the poor oral bioavailability of many ginsenosides is critical for their clinical success. Future research should prioritize pharmaceutical optimization strategies, including structural modification of the ginsenoside backbone to enhance metabolic stability and permeability, the development of advanced nanoformulations (e.g., liposomes or polymeric nanoparticles) for improved solubility and targeted delivery, and the exploration of prodrug approaches to facilitate intestinal absorption. Success in these areas will be pivotal for achieving therapeutic systemic concentrations and maximizing the clinical potential of ginsenosides.
Conclusions
Extensive experimental studies have demonstrated that various ginsenoside monomers can exert anti-HCC effects through multiple pathways. However, current research on the mechanisms of individual ginsenosides in HCC treatment remains insufficiently comprehensive, with their primary signaling pathways and molecular targets not yet fully elucidated. Clinically, while GRg3 combined with TACE shows promise in advanced HCC, key challenges persist: most ginsenosides have poor oral bioavailability, and large-scale placebo-controlled RCTs for HCC are scarce. Future research directions should include developing targeted delivery systems (e.g., nanoparticles, liposomes) and combining ginsenosides with sorafenib or immune checkpoint inhibitors to realize their preclinical potential.
By systematically addressing these translational challenges, ginsenosides are poised to transition from promising preclinical agents to integral components of personalized, multi-targeted HCC treatment regimens in the clinic.
Declarations
Acknowledgement
The schematic diagrams in this article were created using templates from BioRender.com. The publication rights have been secured through a subscription license (Created in BioRender. Luan C. (2025)
Funding
No funding was received for conducting this study.
Conflict of interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors’ contributions
CHL is the sole author of the manuscript.

 Author information
Author information