Introduction
The neurogenic niche represents a highly specialized and dynamic microenvironment critical for supporting neurogenesis—the lifelong process of generating new neurons. This niche comprises diverse elements, including extracellular matrix components, blood vessels, and various secreted factors, which collectively regulate the proliferation, differentiation, and survival of neural stem cells (NSCs).1,2 Disruptions in this finely tuned niche, whether due to aging, injury, or disease, can significantly impair neurogenesis, leading to cognitive dysfunction.3,4 Encouragingly, recent research suggests that rejuvenating the neurogenic niche offers a promising strategy to mitigate these impairments and restore cognitive function, particularly in aging and neurodegenerative disease models.
Adult neurogenesis is a fundamental mechanism underlying brain plasticity, enabling essential cognitive processes such as learning, memory, and emotional regulation. However, its capacity declines progressively with age, contributing to the cognitive deficits commonly observed in older populations.5 As a result, there is growing interest in understanding how neurogenesis can be enhanced to counteract age-related cognitive decline and in developing innovative therapeutic strategies for neurodegenerative diseases. Recent advances in neurobiology have elucidated the intricate regulatory mechanisms governing neurogenesis across various brain regions, highlighting its complex interplay with both intrinsic genetic factors and extrinsic environmental influences.
This review synthesizes current knowledge on the regulation and functional significance of adult neurogenesis, focusing on the cellular and molecular mechanisms underlying its decline with aging and its contribution to cognitive impairment. It examines how aging disrupts neurogenesis in key regions such as the subventricular zone (SVZ) and hippocampus, where NSCs exhibit reduced proliferation and differentiation due to alterations in intrinsic signaling pathways. Additionally, it explores the molecular factors and epigenetic alterations that regulate neurogenesis and how their decline with age contributes to cognitive dysfunction. Methodological challenges and technical limitations in studying neurogenesis are also discussed. Finally, this review underscores the therapeutic potential of targeting neurogenesis as a strategy to enhance cognitive function and promote overall brain health.
Region-specific mechanisms and functional significance of adult mammalian neurogenesis
In rodents, neurogenic activity is primarily localized to two regions: the ventricular-subventricular zone (V-SVZ) and the subgranular zone (SGZ) of the hippocampus. The V-SVZ generates interneurons that contribute to olfactory function, while the SGZ produces granule neurons that integrate into hippocampal circuits essential for memory formation and cognitive processing.6 The functional significance of neurogenesis in rodents is well-established, with its origins tracing back to the earliest discoveries of rodent NSCs in the 1950s.7,8 Studies utilizing transgenic mice, zebrafish, and human brain organoids provide valuable insights into the molecular and cellular mechanisms regulating neurogenesis under both physiological and pathological conditions.9,10
NSCs are integral to adult mammalian neurogenesis, facilitating the generation of neurons and glial cells that are crucial for brain plasticity and repair.11 While NSCs possess self-renewal and multipotent capabilities, their ability to sustain neurogenesis throughout adulthood remains an open question requiring further investigation. Similarly, the extent of their contribution to resilience against neurological and neuropsychiatric disorders is not yet fully understood, necessitating additional research to elucidate their role.
In the adult brain, NSCs are primarily localized within two distinct neurogenic niches: the V-SVZ and the hippocampal SGZ. In the SVZ, NSCs predominantly differentiate into oligodendrocytes and inhibitory interneurons, which migrate along the rostral migratory stream to the olfactory bulb, where they integrate into existing neural circuits. Conversely, NSCs in the SGZ generate excitatory granule neurons within the dentate gyrus. These neurons are critical for cognitive processes such as learning and memory, highlighting the functional importance of NSC activity in this region.6,12,13 These region-specific neurogenic processes are tightly regulated by intrinsic molecular mechanisms.
A detailed comparative overview of hippocampal and SVZ neurogenesis, including cellular stages, markers, and functional significance, is provided in Table 1.14–36
Cellular stages and characteristics of hippocampal and subventricular zone (SVZ) neurogenesis
| Region | Stage | Cell type | Characteristics | Markers | Function/significance | References |
|---|---|---|---|---|---|---|
| Hippocampal Neurogenesis (AHN) | Neural Stem Cells | Type 1 Cells | Origin in the subgranular zone (SGZ); radial apical processes connecting to vasculature; high self-renewal potential; distinguishable from astrocytes by absence of S100β | GFAP, Sox2, Nestin | Serve as the primary source of neurogenesis; retain multipotent capacity for generating neuronal precursors | 14,15 |
| Neuronal Precursors | Type 2 Cells | Arise from asymmetric division of type 1 cells; exhibit high proliferative capacity | GFAP, Sox2, Nestin (Type 2A); DCX, Prox1, NeuroD1, PSA-NCAM (Type 2B) | Transition phase; progressively lose radial morphology and adopt neuronal lineage characteristics | 16 | |
| Type 2A Cells | Retain radial processes; exhibit stem-like properties and high proliferative capacity | GFAP, Sox2, Nestin, Ki67 | Reflect early commitment to proliferation and lineage-specific differentiation | 17,18 | ||
| Type 2B Cells | Downregulate stem-like markers; adopt tangential processes; express neuronal lineage-specific markers | DCX, Prox1, NeuroD1, PSA-NCAM | Mark transition toward neuronal commitment; important for subsequent neuronal differentiation | 14,18 | ||
| Immature Neurons | Type 3 Cells | Radial migration within the SGZ; re-establish apical processes; exit the cell cycle | NeuN, Calretinin | Critical for establishing early neuronal circuits; initiate integration into hippocampal networks | 16,19,20 | |
| Dentate Granule Cells | Immature DGCs | Undergo post-mitotic maturation; somas migrate to the inner granule cell layer; apical dendrites elongate; establish synapses | NeuN, Calretinin (early phase); Calbindin (late phase) | Play a pivotal role in hippocampal functions such as learning and memory; participate in glutamatergic neurotransmission | 21,22 | |
| Mature DGCs | Fully integrated into hippocampal circuits; complete dendritic and synaptic development | Calbindin | Essential for sustaining hippocampal plasticity and cognitive processes | 23,24 | ||
| SVZ Neurogenesis | Neural Stem Cells | Type B1 Cells | Located in the SVZ; exhibit radial glial-like morphology; contact the ventricle and blood vessels; quiescent or slowly dividing | GFAP, Sox2, Nestin, EGFR | Serve as the primary source of neurogenesis; generate intermediate progenitor cells and astrocytes | 25,26 |
| Activated Neural Stem Cells | - | Transition from quiescent Type B1 cells to an activated state; initiate division to produce transit-amplifying cells | GFAP, Sox2, Nestin, Mash1 (Ascl1), Ki67 | Reflect early activation of neurogenesis and commitment to neuronal or glial lineages | 27,28 | |
| Transit-Amplifying Cells | Type C Cells | Rapidly dividing intermediate progenitors; lose radial morphology; generate neuroblasts | Mash1 (Ascl1), Dlx2, Ki67 | Bridge neural stem cells to neuroblast formation; amplify the progenitor pool | 29,30 | |
| Neuroblasts | Type A Cells | Immature neurons; migrate tangentially along the rostral migratory stream (RMS) toward the olfactory bulb; highly motile | DCX, PSA-NCAM, NeuroD1 | Committed to neuronal differentiation; migrate to final destination in the olfactory bulb | 31,32 | |
| Immature Neurons | Type A (Post-Migratory) | Exit the RMS; settle in the olfactory bulb; begin to differentiate further into interneurons | DCX, NeuN (early), Tuj1 | Initiate integration into olfactory bulb circuits; acquire mature neuronal properties | 33,34 | |
| Mature Interneurons | Olfactory Interneurons | Fully differentiated neurons; integrate into the olfactory bulb circuitry; exhibit subtype-specific phenotypes such as granule cells or periglomerular cells | NeuN, Calretinin, Calbindin | Play key roles in olfactory processing, learning, and memory; maintain olfactory bulb plasticity | 35,36 |
Functional studies have highlighted the critical role of neurogenesis in hippocampal function, particularly in learning, memory, and emotional regulation. Disruptions in neurogenesis impair these cognitive functions, whereas factors such as physical exercise, environmental enrichment, and cognitive training promote neurogenesis and enhance cognitive performance.37,38 In contrast, stress, aging, and neurodegeneration negatively affect neurogenesis, contributing to cognitive decline.39 The hippocampus serves as a key region linking neurogenesis to cognitive function (Table 2),40–56 as evidenced by reduced neurogenesis in cyclin D2-deficient mice, which correlates with impaired sleep-dependent memory consolidation.40 This underscores the hippocampus’s pivotal role in memory processing. Additionally, molecular regulators such as Capicua and Dagla emphasize the importance of transcriptional and signaling pathways in sustaining neurogenic potential. Furthermore, dysregulated cannabinoid-mediated signaling pathways are implicated in age-related cognitive decline,57,58 further highlighting the significance of molecular mechanisms in maintaining brain function.
Neurogenesis across brain regions: Key findings and implications for aging and disease
| Topic | Key findings | Implications | References |
|---|---|---|---|
| Hippocampal neurogenesis | In an animal study, Cyclin D2-deficient mice exhibit impaired sleep-dependent memory consolidation due to neurogenic decline | Neurogenic decline contributes to sleep and memory disturbances in aging | 40,41 |
| V-SVZ neurogenesis | A human study found that neurogenesis in the ventricular-subventricular zone (V-SVZ) declines early due to neuroinflammation | Targeting neuroinflammation could preserve neurogenesis and cognitive function | 42 |
| Hypothalamic neurogenesis | In molecular and animal studies, unsaturated fatty acids promote neurogenesis via brain derived neurotrophic factor (BDNF) signaling in rodents | Promotes metabolic homeostasis, suggesting therapeutic potential for aging | 43,44 |
| Cannabinoid signaling | A molecular study showed that the Dagla enzyme facilitates NSPC proliferation via autocrine signaling pathways | Disruptions in cannabinoid signaling accelerate age-related neurogenic decline | 45,46 |
| External influences (IH, T2DM) | An animal study revealed that IH (sleep apnea) and T2DM impair neurogenesis through oxidative stress | Antioxidants, exercise, and therapeutic compounds can mitigate neurogenic loss | 47 |
| NG2 cell reprogramming | Animal studies demonstrated that NG2 progenitors in cortical regions exhibit location-dependent plasticity | Offers potential for neuronal reprogramming to repair cortical damage | 48–50 |
| Zebrafish models | In an animal study, Pink1-deficient zebrafish exhibit reduced dopaminergic neurogenesis, modeling age-related neurogenic decline and Parkinson’s pathology | Models age-related neurogenic decline and Parkinson’s pathology | 51,52 |
| Striatal neurogenesis | Animal studies show that striatal regions exhibit neural progenitor activity in response to injury | Potential for neuronal repair in movement disorders and injury-related damage | 53 |
| Olfactory bulb neurogenesis | In molecular and animal studies, the olfactory bulb shows significant neurogenesis in certain species | Implications for sensory processing, olfactory memory, and aging | 54 |
| Cerebellar neurogenesis | Animal studies indicate that the cerebellum maintains progenitor activity, particularly after injury | Possible therapeutic strategy for motor coordination disorders and cerebellar damage | 55 |
| Prefrontal cortex neurogenesis | In an animal study, limited neurogenesis is observed in the prefrontal cortex of aged mammals | Targeting neurogenesis in this region may enhance cognitive functions in aging | 56 |
Hippocampal neurogenesis begins with the activation of quiescent NSCs, or radial glia-like cells. These cells divide asymmetrically to produce intermediate progenitor cells, which quickly differentiate into neuroblasts. These neuroblasts mature into functional neurons that integrate into the hippocampal circuits.59 Symmetric division of NSCs ensures self-renewal, generating additional quiescent NSCs. However, a significant proportion of newly formed neurons undergo apoptosis before fully integrating into the dentate gyrus,60 highlighting the strict regulation of neurogenesis. This complexity presents challenges for therapeutic strategies aimed at enhancing NSC activity for brain repair and regeneration.
In the SVZ, neurogenesis follows a similar initiation process. Quiescent NSCs, or radial glia-like cells, are activated and undergo asymmetric division to produce progenitor cells. These progenitor cells proliferate and differentiate into neuroblasts, which migrate along the rostral migratory stream to the olfactory bulb. There, they integrate into the existing neural circuits as interneurons.1 Although apoptosis also occurs during SVZ neurogenesis, it is believed to be crucial for maintaining the quality of the new neuronal population.61 The newly generated neurons in the olfactory bulb contribute to sensory processing, specifically olfactory memory and odor recognition.62 Despite similarities in processes such as asymmetric division and neuroblast integration, hippocampal and SVZ neurogenesis differ in their functional roles. Hippocampal neurogenesis supports memory formation and learning, whereas SVZ neurogenesis primarily contributes to olfactory memory processing. Both regions exhibit high rates of apoptosis during neurogenesis, but the migration process in the SVZ adds another layer of complexity. While exercise and environmental enrichment enhance neurogenesis in the hippocampus, their impact on SVZ neurogenesis remains less understood (Table 3).39,63–87 These differences reflect the distinct functional contributions of the hippocampus and SVZ to brain plasticity and cognitive function.
Comparative functional roles of hippocampal adult-born dentate granule cells (abDGCs) and newborn olfactory sensory neurons (OSNs) in memory processing and cognitive flexibility
| Concept | Hippocampal adult-born neurons (abDGCs) | Newborn olfactory neurons (OSNs) | References |
|---|---|---|---|
| Functional Properties | Studies utilizing animal and molecular approaches demonstrate that abDGCs exhibit hyperexcitability and reduced GABAergic inhibition during maturation, facilitating specialized roles in learning and memory | Animal and molecular studies indicate that newborn OSNs in the olfactory epithelium exhibit high plasticity, enabling their integration into existing olfactory circuits for odor detection and memory | 63,64 |
| Pattern Separation | Animal and molecular studies reveal that abDGCs enhance the dentate gyrus’s ability to distinguish similar inputs, thereby reducing memory interference through sparse neural signaling | Evidence from animal and molecular studies suggests that newborn OSNs contribute to distinguishing overlapping odors, supporting olfactory pattern separation and memory formation | 64–66 |
| Synaptic Competition & Memory Discrimination | Animal and molecular studies indicate that abDGCs compete with mature dentate granule cells for synaptic connections, facilitating synapse modulation or elimination and improving memory discrimination | Research employing animal and molecular methodologies shows that newborn OSNs integrate into the olfactory bulb and compete with existing neurons, supporting sensory memory updates and olfactory context discrimination | 63,67,68 |
| Temporal Encoding | Findings from animal and molecular studies suggest that the hyperexcitability of abDGCs enables them to associate distinct memory events over time, effectively “timestamping” memories | Experimental data from animal and molecular studies demonstrate that newborn OSNs adapt to new odors and temporal cues, contributing to the temporal encoding of sensory inputs and memory organization | 69,70 |
| Role in Cognitive Flexibility | Animal and molecular studies support the role of adult hippocampal neurogenesis in enhancing cognitive flexibility by promoting adaptability, allowing the brain to update and retain specific memories | Research utilizing animal and molecular approaches suggests that newborn OSNs contribute to olfactory system adaptability, facilitating recovery from sensory impairments and responsiveness to novel sensory inputs | 71,72 |
| Complementary Roles of Immature & Mature Neurons | Studies employing animal and molecular techniques reveal that immature abDGCs facilitate pattern separation, while mature DGCs contribute to pattern completion, thereby supporting memory retrieval | Animal and molecular studies suggest that immature OSNs integrate into olfactory circuits, while mature neurons maintain sensory memory and perception stability | 63,66,67,73 |
| Exercise-Induced Enhancements | Animal and molecular studies indicate that exercise-induced neurogenesis enhances pattern separation but may impair the retention of prior memories due to accelerated memory clearance | Evidence from animal and molecular research suggests that exercise and environmental enrichment stimulate newborn OSN integration, improving odor perception and learning | 74–77 |
| Role in Memory Formation | Studies employing animal and molecular methodologies demonstrate that abDGCs enhance spatial and contextual memory encoding and learning by continuously updating and refining memory representations | Animal and molecular research highlights the role of newborn OSNs in sustaining olfactory memory by ensuring consistent sensory input and facilitating odor-based learning and memory retrieval | 63,66,78,79 |
| Implications for Cognitive Disorders | Both animal and human studies suggest that disruptions in adult hippocampal neurogenesis are associated with cognitive disorders, including memory deficits and neurodegenerative diseases | Findings from animal and human studies indicate that disruptions in newborn OSN generation contribute to olfactory dysfunction, often an early indicator of neurodegenerative diseases | 80–82 |
| Therapeutic Potential | Animal and human studies propose that targeting adult hippocampal neurogenesis pathways may offer novel therapeutic strategies for addressing memory impairments, cognitive flexibility deficits, and neurodegenerative diseases | Evidence from animal and human studies suggests that stimulating newborn OSN generation could enhance olfactory memory and function, potentially aiding in the recovery of olfactory deficits associated with aging and disease | 83–86 |
| Function in Aging & Neurodegeneration | Research incorporating both animal and human models indicates that the age-related decline in adult hippocampal neurogenesis is associated with memory loss and cognitive decline, making it a critical target for interventions against age-related memory deficits | Animal and human studies suggest that the decline in newborn OSN generations during aging and neurodegeneration contributes to olfactory dysfunction, potentially leading to broader cognitive impairments | 39,80,87 |
External factors and systemic influences also modulate neurogenesis and cognitive outcomes. For instance, intermittent hypoxia—commonly caused by sleep apnea—impairs neurogenesis through oxidative stress.88 Similarly, metabolic disorders such as type-2 diabetes mellitus reduce hippocampal neurogenesis and exacerbate cognitive deficits.89 Despite these challenges, interventions such as antioxidant therapies, physical exercise, and pharmacological agents (e.g., andrographolide) have shown promise in reversing neurogenic decline and improving cognitive function.90 These findings suggest that targeted therapies could restore neurogenic capacity and promote brain health in aging and disease.
Beyond the hippocampus and V-SVZ, neurogenesis in regions like the hypothalamus provides critical insights into broader mechanisms of neural regeneration (Table 2).91,92 While most adult neurogenesis research has focused on the hippocampus and V-SVZ, emerging evidence suggests that neurogenesis also occurs in other brain regions, including the olfactory bulb,54 striatum,53 and hypothalamus.43,44 This expanded understanding points to the potential for neuronal repair and regeneration throughout the adult brain, opening new avenues for therapeutic intervention.
In humans, neurogenesis in the V-SVZ declines early, often due to neuroinflammation.93 This highlights the need for further investigation into this early decline and the potential for targeting this region to enhance neurogenesis in aging, neurodegenerative diseases, stroke, or brain injury. Meanwhile, neurogenesis in the hypothalamus, modulated by factors such as unsaturated fatty acids and neurotrophic signaling, plays a key role in maintaining metabolic homeostasis.43,94 This suggests broader implications for systemic health and the potential for treating both metabolic and neurodegenerative diseases simultaneously. Additionally, studies using zebrafish models and cortical NG2 cells emphasize the possibility of reprogramming progenitor cells to facilitate neuronal repair in aging or damaged brains.50,95 Emerging research also indicates that neurogenesis in other regions, such as the striatum, may be involved in motor function and repair following injury. Similarly, the olfactory bulb, which exhibits robust neurogenesis in certain species, may provide valuable insights into sensory processing and olfactory memory in aging and neurodegenerative diseases. This broader view of neurogenesis challenges the traditional perspective of it being a localized process, emphasizing the complexity and adaptability of the adult brain.
The impact of aging on neural stem cell function and neurogenesis: Mechanisms and consequences
Aging profoundly affects NSC function and regenerative capacity, leading to a notable decline in adult neurogenesis. This age-related decline results from a combination of intrinsic and extrinsic factors that disrupt the balance of neurogenic processes.96 Intrinsic factors, such as reduced NSC proliferation and epigenetic drift, contribute significantly to this decline. Epigenetic drift refers to age-associated alterations in gene regulation that shift NSC behavior from self-renewal toward differentiation, ultimately depleting the NSC pool over time.97,98 In addition to these intrinsic changes, extrinsic factors—including impaired growth factor signaling, elevated corticosteroid levels, and cellular senescence—further compromise NSC function and neurogenesis.99 Elevated glucocorticoid levels, common in aging, are particularly known to inhibit NSC proliferation, possibly through epigenetic mechanisms.100 However, their direct role in influencing NSC differentiation remains poorly understood and warrants further investigation.
Aging induces both structural and functional changes in the brain, often manifesting as cognitive decline.101 Adult neurogenesis plays a crucial role in maintaining brain plasticity, supporting critical functions such as learning, memory, and emotional regulation. As aging disrupts the regulatory pathways controlling neurogenesis, the resulting decline in neurogenic activity becomes a major contributor to cognitive dysfunction.39,102 The disruption of these pathways is multifaceted, involving the interplay of genetic, molecular, and environmental factors. Recent advances in experimental models, including genetic manipulations in rodents and the use of human brain organoid systems, have provided deeper insights into the molecular mechanisms regulating neurogenesis.103,104 These models have also highlighted the consequences of neurogenesis decline across species and under various pathological conditions, offering valuable clues for potential therapeutic interventions.
Despite the substantial decline in neurogenesis with aging, NSCs in the aged brain retain partial functionality. In vitro studies have shown that NSCs derived from aged rodents can still proliferate and differentiate, though less efficiently compared to those from younger counterparts.96,105 This suggests that the neurogenic niche becomes less conducive to NSC activation and neurogenesis with age. However, neurons derived from aged NSCs can still integrate into existing neural circuits, suggesting the potential for therapeutic strategies aimed at rejuvenating NSCs. Understanding age-related changes in NSC dynamics and developing interventions to restore the neurogenic niche could help mitigate cognitive decline and enhance brain repair.
Adult neurogenesis in rodents and humans: Mechanisms, and challenges in understanding its role in cognitive function
Adult neurogenesis has been extensively studied in rodents and non-human primates in recent decades. Initially identified in rodents, this phenomenon has since been observed in humans, with accumulating evidence suggesting its persistence throughout life, particularly in the hippocampus.102,106,107 This ability to generate neurons in the adult brain plays a pivotal role in maintaining cognitive function and brain plasticity. Despite challenges in fully understanding neurogenesis dynamics in humans, it has been linked to processes such as learning, memory, and emotional regulation.102,108
In rodents, adult neurogenesis progresses through a series of distinct, interdependent stages over approximately seven weeks, a process extensively characterized in the hippocampus.106,109 These stages involve complex molecular, cellular, and physiological transformations that ultimately lead to the production of fully functional neurons capable of integrating into neural circuits. This process is essential for brain plasticity, learning, and memory and holds considerable therapeutic potential for treating disorders associated with impaired neurogenesis,110 such as neurodegenerative diseases and cognitive decline.5,111 A comprehensive understanding of these stages is critical for developing clinical strategies aimed at enhancing neurogenesis (Table 4).109,112–126
Cellular and molecular dynamics of neurogenesis: Stages, functional implications, and therapeutic insights
| Stage | Cellular and molecular events | Functional implications | Therapeutic insights | References |
|---|---|---|---|---|
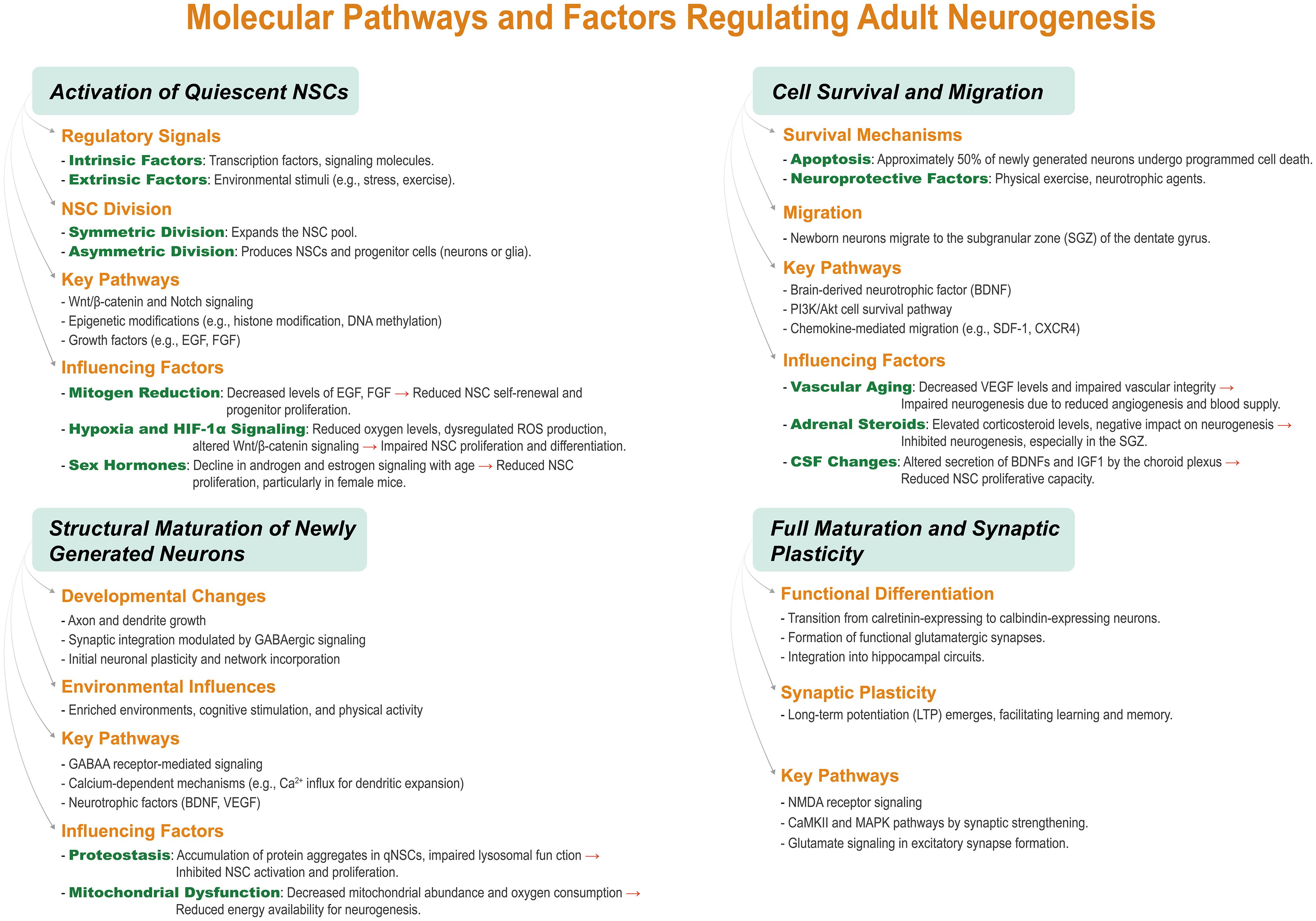
| Precursor Cell Activation | Quiescent neural stem cells (NSCs), resembling astrocytes, become activated in response to intrinsic (e.g., signaling molecules) and extrinsic (e.g., environmental stimuli) cues. Molecular and animal studies indicate that activated NSCs undergo symmetric division to expand the NSC pool or asymmetric division to generate progenitor cells, which differentiate into neurons or glial cells. Migration towards the subgranular zone (SGZ) of the dentate gyrus (DG) occurs at this stage | This activation is the foundational event in neurogenesis. It establishes the pool of NSCs necessary for continued neurogenesis. If disrupted, this step could result in insufficient neuronal production, contributing to cognitive impairments, such as those observed in aging and neurodegenerative diseases | Manipulating NSC activation pathways could be a key strategy in conditions like Alzheimer’s disease, where neurogenesis is impaired. Enhancing NSC activation might increase the pool of potential neurons, aiding in cognitive recovery or preventing further neuronal loss | 112–115 |
| Early Survival | Following activation, approximately 50% of newly generated cells undergo apoptosis, a selective process ensuring that only functional neurons persist. Animal studies reveal that surviving cells migrate towards the granule cell layer (GCL) of the DG, where they extend axons and dendrites. This stage marks the transition from proliferation to differentiation, during which cells exhibit high plasticity and begin integrating into neural circuits | This stage is critical for the refinement of neurogenesis, ensuring only the most functionally viable neurons survive. It also facilitates the early structural development of neurons, including the extension of axons and dendrites, essential for synaptic integration and network formation | Interventions to enhance cell survival may be beneficial in promoting neurogenesis in individuals with neurodegenerative diseases or those experiencing stress-induced hippocampal dysfunction. Strategies like neuroprotective agents or exercise could enhance neuronal survival | 109,116–119 |
| Early Postmitotic Maturation | Newly formed neurons undergo rapid structural maturation, including axonal elongation, dendritic growth, and dendritic spine formation. Molecular and animal studies highlight the crucial role of GABAergic signaling in synaptic connection formation. While still immature, these neurons begin exhibiting initial synaptic activity as they integrate into hippocampal circuits | The maturation of axons and dendrites during this phase is crucial for synaptic formation, making the neurons capable of receiving and sending signals within neural circuits. The neurons remain highly plastic, essential for learning and memory processes | Environmental factors, such as enriched environments, exercise, and cognitive stimulation, could accelerate this maturation process, improving cognitive outcomes and promoting faster integration of new neurons into functional circuits. This stage is a target for non-invasive interventions | 120–122 |
| Late Maturation | Newly formed neurons acquire mature electrophysiological properties, transitioning from expressing calretinin to calbindin, which signifies full maturation. Molecular, animal, and human studies confirm the formation of functional glutamatergic synapses, facilitating excitatory neurotransmission. Synaptic plasticity, including long-term potentiation (LTP), emerges, supporting learning and memory processes | Mature neurons contribute to the hippocampal network, participating in complex processes like memory consolidation and learning. The transition to synaptic plasticity allows for the storage of information, while the neurons become fully functional components of the hippocampal circuit | Enhancing synaptic plasticity during this stage could provide therapeutic benefits, particularly for aging populations or individuals with memory disorders. Promoting LTP or synaptic connectivity could improve cognitive function and memory formation in neurodegenerative diseases | 123–126 |
The first crucial step in neurogenesis is the activation of NSCs. Disruptions to this activation, such as those caused by aging or neurodegenerative diseases, can impair neurogenesis.127,128 Therapeutic approaches focused on enhancing NSC activation could provide potential treatments for cognitive decline and neurodegenerative conditions by boosting neurogenesis. Additionally, new neurons undergo selective survival, ensuring that only those capable of integrating into existing neural circuits persist.129,130 Interventions such as exercise or pharmacological agents may increase the survival rate of these neurons (Fig. 1),131 offering promising strategies to combat cognitive impairments observed in diseases like Alzheimer’s or depression.
During the early postmitotic maturation phase, neurons undergo structural maturation, including synaptic formation and integration into functional networks. Environmental factors, such as physical activity and enriched environments, can accelerate neuronal maturation, leading to improved cognitive outcomes.5,132,133 Enhancing this stage may serve as a therapeutic strategy to improve memory and learning functions in individuals with neurogenic impairments. The final maturation phase involves the acquisition of electrophysiological properties and synaptic plasticity, both of which are essential for learning and memory. Enhancing synaptic plasticity or long-term potentiation could improve cognitive performance in individuals experiencing age-related cognitive decline or neurodegenerative diseases.125,126,134
While adult neurogenesis is well-documented in rodents, confirming its occurrence in humans has proven more challenging. Several techniques, including bromodeoxyuridine (BrdU) incorporation, immunohistochemistry for immature neuron markers, and carbon-14 DNA dating, have been employed to study neurogenesis in the human brain.107,135,136 One pioneering study demonstrated new neuron generation in the human hippocampus by labeling proliferating cells with BrdU, showing that these BrdU-positive cells expressed neuronal markers, confirming the formation of new neurons in the hippocampal dentate gyrus.137
Despite these findings, accurately estimating the extent of neurogenesis in humans remains difficult. A study using radiocarbon dating suggested that neurogenesis in the human hippocampus occurs at an annual turnover rate of approximately 1.75%.138,139 However, other studies have failed to detect significant new neuron generation,102 leading to debates about the true extent of neurogenesis in humans. Inconsistencies in reported adult human neurogenesis arise from variations in methodology, sample processing, and the sensitivity of detection techniques. While some studies provide evidence of robust neurogenesis, others suggest that new neuron formation is minimal or absent. Factors contributing to these conflicting findings include differences in tissue preparation, postmortem interval, fixation procedures, and the selection of neuronal markers. Furthermore, the sensitivity and specificity of detection techniques, such as immunohistochemistry and in situ hybridization, play a crucial role in accurately identifying newly formed neurons.
Beyond methodological challenges, the decline of neurogenesis with age further complicates efforts to assess its prevalence in older individuals. Age-related reductions in neural progenitor proliferation, diminished availability of growth factors, and increased inflammatory signaling within the brain microenvironment are all contributing factors. Moreover, systemic influences, such as chronic stress, metabolic disorders, and neurodegenerative diseases, have been implicated in modulating neurogenesis rates, highlighting the complexity of interpreting findings across different studies.
Technical limitations also contribute to the observed discrepancies. Traditional methods, such as BrdU incorporation and carbon-14 dating, face challenges in distinguishing neuronal turnover from other forms of cell proliferation. Emerging technologies, including single-cell RNA sequencing and lineage-tracing approaches, offer more precise insights into neurogenic processes and hold promise for reconciling conflicting findings in the field. These methodological challenges, biological factors, and technical limitations are discussed in subsequent sections.
Taken together, these discrepancies suggest that neurogenesis rates may vary depending on factors such as age, health status, and tissue preservation methods. Resolving these uncertainties will require future research to adopt standardized methodologies, conduct longitudinal studies, and utilize advanced imaging techniques. Such efforts will be essential in elucidating the functional significance of adult neurogenesis in humans and its broader implications for neurological health and disease.
Decline of adult hippocampal neurogenesis: Intrinsic and extrinsic factors influencing NSC activity in aging
Adult neurogenesis, particularly in the hippocampus, plays a crucial role in maintaining cognitive functions such as memory and learning. While the biological significance of neurogenesis is well-established in some species, its exact role in human cognition remains a subject of ongoing research. As individuals age, the number of NSCs in both the SGZ and the V-SVZ decreases, leading to a decline in neurogenesis.140,141 This reduction is influenced by a combination of intrinsic and extrinsic factors, particularly changes in signaling pathways that regulate NSC activity (Table 5).142–164 Key mitogens, such as epidermal growth factor and fibroblast growth factor, which are critical for NSC self-renewal and the proliferation of transit-amplifying progenitor cells (TAPs), diminish with age, significantly contributing to the decline in neurogenesis.142,165
Factors influencing neurogenesis: Mechanisms and impact on NSC function
| Factor | Mechanism | Impact on neurogenesis | References |
|---|---|---|---|
| Mitogen Reduction | Molecular and animal studies have demonstrated that reduced levels of EGF and FGF in NSCs lead to impaired signaling pathways necessary for cell proliferation | Reduced NSC self-renewal and progenitor cell proliferation | 142–144 |
| Vascular Aging | Animal and human studies indicate that aging is associated with decreased VEGF levels and impaired vascular integrity, leading to reduced angiogenesis and cerebral blood flow | Impaired neurogenesis due to reduced angiogenesis and blood supply | 145–147 |
| Proteostasis | Molecular studies have revealed that the accumulation of protein aggregates in quiescent NSCs, coupled with impaired lysosomal function, disrupts intracellular homeostasis | Inhibited NSC activation and proliferation | 148,149 |
| Mitochondrial Dysfunction | Molecular and animal studies suggest that mitochondrial dysfunction, characterized by decreased mitochondrial abundance and oxygen consumption in NSCs, leads to metabolic insufficiency | Reduced energy availability for neurogenesis | 150–152 |
| Hypoxia and HIF-1α Signaling | Findings from molecular and animal studies indicate that reduced oxygen levels, dysregulated ROS production, and altered Wnt/β-catenin signaling impair cellular responses in NSCs | Impaired NSC proliferation and differentiation | 153–155 |
| Sex Hormones | Animal and human studies have shown that aging-related declines in androgen and estrogen signaling alter neurogenic processes | Reduced neurogenesis, particularly in female mice | 156–158 |
| Adrenal Steroids | Animal studies indicate that elevated corticosteroid levels exert negative effects on hippocampal NSCs by disrupting neurogenic signaling pathways | Inhibited neurogenesis, especially in the SGZ | 159–161 |
| CSF Changes | Molecular and animal studies have demonstrated that altered secretion of BMP5 and IGF1 by the choroid plexus affects NSC function | Reduced NSC proliferative capacity | 162–164 |
Epigenetic modifications, including alterations in DNA methylation, have been implicated in the decline of neurogenesis. A reduction in the expression of the DNA demethylase enzyme ten-eleven translocation methylcytosine dioxygenase 2 has been linked to decreased neurogenesis in the SGZ.166,167 Interestingly, heterochronic parabiosis studies have shown that restoring translocation methylcytosine dioxygenase 2 expression in aged hippocampi can promote neurogenesis.140,168 The microenvironment surrounding NSCs, particularly the vasculature, also plays a pivotal role in regulating NSC activity. Vascular endothelial growth factor, a glycoprotein that promotes angiogenesis, positively influences neurogenesis. However, with aging, vascular endothelial growth factor levels in the hippocampus decrease, contributing to reduced neurogenesis.169–171 Additionally, cerebral blood volume in the dentate gyrus correlates with neurogenesis,172,173 suggesting that vascular aging impairs NSC function.
Proteostasis is another essential factor in maintaining NSC activity. Aging leads to the accumulation of protein aggregates in quiescent NSCs, which are stored in lysosomes and impede their activation and proliferation. Enhancing the lysosomal pathway in aged quiescent NSCs has been shown to improve their activation and neurogenesis.174,175 Mitochondrial dysfunction is also a contributing factor, as aged progenitor cells in the SGZ exhibit reduced mitochondrial abundance and oxygen consumption, limiting neurogenesis.39,152,152 Although mitochondrial DNA mutation rates do not significantly increase in aged neural progenitor cells, mitochondrial dysfunction remains a critical factor in diminished neurogenesis.
Sex hormones such as androgens and estrogens are known regulators of adult neurogenesis. Androgens positively influence neurogenesis in the SGZ of male mice, but their levels decline with age, reducing the sensitivity to androgen signaling. Estrogen promotes neurogenesis in young female rats, although this effect diminishes with age.156–158 Conversely, adrenal steroids, including corticosteroids, negatively impact neurogenesis, particularly in the SGZ.159 Reducing corticosteroid levels through adrenalectomy has been shown to enhance neurogenesis in aged mice.176 Cerebrospinal fluid (CSF), produced by the choroid plexus, contains signaling molecules that change with age. NSCs in the V-SVZ interact with CSF, and age-dependent changes in the secretome of the lateral ventricular choroid plexus can influence NSC regulation.164,177,178 Decreased secretion of bone morphogenetic protein 5 and insulin-like growth factor 1 from the choroid plexus in aged individuals reduces NSC proliferative capacity, further contributing to the decline in neurogenesis.179,180
Reactivating dormant NSCs to promote neurogenesis holds significant promise for regenerative medicine, particularly for treating neural loss due to cerebrovascular disorders, traumatic brain injury, and neurodegenerative diseases.181 However, transient increases in neurogenesis caused by NSC activation may eventually deplete the NSC pool, limiting long-term regenerative potential. For example, in epilepsy models, repeated seizures stimulate NSCs, temporarily increasing neurogenesis but eventually leading to NSC depletion and neuronal loss.182 Maintaining sustained neurogenic responses remains a challenge, but recent studies suggest that the expression of p38 mitogen-activated protein kinase in the V-SVZ may support long-term neurogenesis without depleting the NSC pool.183 Additionally, amplifying and mobilizing TAPs could provide a more sustainable approach for brain repair.140
Age-related decline and pathological impairments in adult neurogenesis: Insights from rodent models to human aging and Alzheimer’s disease (AD)
Age-related decline in adult neurogenesis: Insights from rodent and human models
Adult neurogenesis in the SVZ and dentate gyrus decline significantly with age, marked by reduced proliferative activity. In rodents, this decline begins as early as six months of age, with a significant reduction observed by 20–24 months. Early studies using BrdU incorporation techniques have demonstrated a dramatic decrease in progenitor cell proliferation, with rates dropping to approximately 10% of adult levels.3,108,184,185 This reduction severely impacts the generation of new granule cells, contributing to age-related cognitive and sensory deficits.
The age-related decline in neurogenesis is further characterized by a shift in the balance between actively dividing and quiescent NSCs. Over time, the NSC pool becomes progressively depleted, with fewer cells remaining in an actively dividing state.96,186 Additionally, the balance between symmetric and asymmetric cell divisions becomes increasingly skewed, limiting the neurogenic potential of the remaining stem cells.187,188 These changes highlight the progressive nature of neurogenic decline and its correlation with functional impairments in rodents.
Comparative studies between rodent and human models reveal both similarities and differences in the decline of neurogenesis. Rodent models provide valuable insights into the mechanisms underlying age-related reductions in neurogenesis, offering a framework to understand the cognitive and sensory deficits associated with aging.5,189 In humans, the decline in neurogenesis is more gradual, influenced not only by aging but also by pathological conditions such as AD.3,190,191
Whether adult neurogenesis continues in aging brains remains a subject of debate, with studies yielding conflicting results. Some reports suggest that neurogenesis persists throughout life, albeit at significantly reduced levels, particularly in the dentate gyrus of the hippocampus.17 However, other studies argue that adult neurogenesis is negligible or absent in older individuals,192 citing a lack of detectable neurogenic markers in postmortem human brains. Methodological differences, including variations in tissue processing, labeling techniques, and age ranges examined, may contribute to these discrepancies. While emerging evidence supports the notion that neurogenesis may continue under specific physiological conditions or in response to environmental stimuli, further research is needed to reconcile these findings and establish a consensus on the extent of neurogenesis in aging humans.
In AD, neurogenesis in the dentate gyrus is significantly impaired, with reduced granule cell maturation and hippocampal dysfunction. Pathological features such as amyloid plaques, tau tangles, and neuroinflammation exacerbate the decline in neurogenic processes.80,193,194 In rodent models of AD, similar neurogenic impairments are observed, including decreased progenitor cell proliferation and maturation,195,196 providing a translational perspective for understanding human neurogenesis in both health and disease.
This comparative analysis underscores the importance of studying neurogenesis across species to elucidate the mechanisms driving age-related and disease-associated declines, with implications for developing targeted therapies to preserve or restore neurogenic capacity (Table 6).3,17,23,38,80,102,107,186,190,192,194,197–205
Comparative analysis of age-related decline in neurogenesis and Alzheimer’s disease impact in rodent and human models
| Factor | Rodent model | Human model | References |
|---|---|---|---|
| Age of Decline in Neurogenesis | Begins at about six months; more pronounced at 20–24 months | Observed as age progresses, with a gradual reduction in DG neurogenesis | 3,107,197 |
| Proliferative Activity | Decreases to 10% of adult levels by 20–24 months | Decreased percentage of immature neurons in DG, with slower incorporation of new neurons. | 3,23,38 |
| NSC Pool | Likely depleted over time; shifts towards symmetric divisions, reducing neurogenic potential | Depletion of quiescent NSC pool in the anterior DG, limiting regeneration potential | 17,186,192,198,199 |
| Exercise Influence | Physical exercise can rescue age-related deficits in neurogenesis, stimulating proliferation and survival of neural progenitor cells (NPCs) | Limited data: some evidence suggests neurogenesis persists in adulthood, but exercise effects remain unclear | 102,200,201 |
| Alzheimer’s Disease Impact | Impairs neurogenesis, granule cell maturation, and reduces hippocampal function | Maturation of DCX-positive neurons is impaired, with limited integration of new neurons in the hippocampus | 3,80,190,194 |
| Pathological Features of AD | Amyloid plaques, tau tangles, and neuronal dysfunction significantly impair neurogenic processes | Presence of amyloid plaques, tau tangles, and neuroinflammation contribute to the decline of neurogenesis, particularly in the hippocampus | 202–205 |
The multifactorial decline of adult neurogenesis with aging: Mechanisms and potential interventions
The decline in adult neurogenesis with aging is a complex, multifactorial process influenced by both intrinsic and extrinsic factors. As age progresses, the production of new neurons decreases markedly, as demonstrated in both rodent models and humans. This reduction results from a combination of cellular, molecular, and environmental changes that collectively impair the brain’s neurogenic capacity over time.96
Physical activity emerges as one of the most effective extrinsic factors in countering age-related declines in neurogenesis, particularly in the hippocampus.200 Exercise promotes the release of neurotrophic factors such as brain-derived neurotrophic factor, which supports neuronal survival, growth, and synaptic plasticity.206 Furthermore, exercise enhances hippocampal blood flow, facilitating nutrient delivery and waste removal that sustains neurogenic processes.207 In rodent studies, voluntary exercise has been shown to increase the proliferation of neural progenitor cells and improve the survival of newly generated neurons in the dentate gyrus, partially mitigating the neurogenic decline associated with aging.208,209
Another extrinsic factor influencing neurogenesis is parabiosis, a process where aged animals are surgically joined with younger ones to share a circulatory system.210,211 Research indicates that exposure to the systemic environment of younger organisms can rejuvenate neurogenic capacity and enhance cognitive function in aged animals. These findings suggest that circulating factors in young blood play a critical role in revitalizing the aging brain, pointing to potential therapeutic strategies aimed at modifying the systemic environment to counteract neurogenic decline.
Intrinsic cellular and molecular changes also significantly impact neurogenesis as the brain ages. Aging disrupts key signaling pathways that regulate NSC self-renewal and differentiation, including bone morphogenetic protein, Notch, Wnt, epidermal growth factor, and insulin-like growth factor.212 These pathways lose efficiency over time, impairing NSC proliferation and differentiation, further diminishing neurogenic output and cognitive function.96,213
The aging brain also experiences a marked decline in proteostasis, the system responsible for maintaining protein quality control. Aged NSCs exhibit reduced autophagy-lysosome activity, leading to the accumulation of damaged proteins and organelles that contribute to cellular stress and dysfunction.214,215 Additionally, chaperone proteins, which assist in proper protein folding and prevent aggregation, show diminished activity in aged NSCs.216,217 These deficits in proteostasis exacerbate the decline in NSC functionality and the generation of new neurons.
Epigenetic modifications further compound the decline of neurogenesis with age. Alterations in DNA methylation, histone modifications, and non-coding RNA expression silence genes essential for neurogenesis while activating those associated with cellular senescence and apoptosis.218,219 These epigenetic changes contribute to the progressive loss of neurogenic potential in the aging brain.
The intricate interplay between intrinsic and extrinsic factors highlights the complexity of neurogenic decline associated with aging. Understanding these mechanisms is essential for designing strategies to restore neurogenic capacity and promote cognitive health.
Neurogenesis in AD: Evolutionary conservation and emerging technologies
AD is a progressive neurodegenerative disorder characterized by memory loss, cognitive decline, and widespread neuronal and synaptic degeneration, particularly affecting the hippocampus and entorhinal cortex.220 The pathological hallmarks of AD include extracellular amyloid plaques and intracellular neurofibrillary tangles, both central to disease progression. Amyloid plaques, primarily composed of amyloid-beta (Aβ), form from the cleavage of amyloid precursor protein by β-secretase and γ-secretase.221 According to the “amyloid hypothesis,” the accumulation and aggregation of Aβ drive the progression of AD, leading to synaptic dysfunction, gliosis, neuronal loss, and tau pathology.222 Concurrently, tau hyperphosphorylation and the formation of neurofibrillary tangles disrupt essential cellular processes, exacerbating neuronal dysfunction and death.223
Neurogenesis is profoundly impaired in AD, particularly within the hippocampus, a region essential for learning and memory. The dentate gyrus of the hippocampus exhibits a marked reduction in immature neurons, with their maturation significantly hindered. Key markers of neuronal maturation, such as doublecortin, are under-expressed, impeding the proper integration of newly generated neurons into neural circuits.224 This disruption in neurogenesis likely contributes to the cognitive deficits characteristic of AD. Although neurogenesis persists into adulthood, its rate in AD patients is significantly lower compared to healthy aging individuals, further exacerbating cognitive decline.3,190,191
The mechanisms underlying impaired neurogenesis in AD are multifactorial. Aβ plaques directly impair the survival and differentiation of NSCs, potentially through the activation of inflammatory pathways and the disruption of neurotrophic signaling.193,225 Tau pathology also plays a critical role by altering the hippocampal microenvironment and further compromising NSC function.226,227 Chronic inflammation and oxidative stress,228 hallmarks of AD, amplify these effects by creating a hostile environment that impedes neurogenesis.
The decline in neurogenesis with aging and its exacerbation in AD remain active research areas. Many signaling pathways and cellular mechanisms involved in neurogenesis are conserved across species, making rodent models invaluable for understanding human neurogenesis. However, species-specific differences must be carefully considered, especially regarding aging and neurodegenerative diseases.
Emerging technologies, such as single-cell genomics, offer unprecedented opportunities to study human adult neurogenesis in detail. These technologies enable the profiling of individual NSCs and their progeny, providing insights into molecular changes during aging and AD. Additionally, innovations in cellular reprogramming and gene editing allow for the generation of patient-specific models of neurodegenerative diseases, facilitating the study of neurogenesis in the context of specific genetic mutations and pathological conditions.
From hippocampal plasticity to neurodegenerative pathology: Exploring the dual role of neurogenesis in health and disease
Neurogenesis plays a critical role in the hippocampus, contributing to memory and cognitive function. However, it is intricately linked to aging and the pathophysiology of neurodegenerative diseases such as AD and Parkinson’s disease (PD).39,191Table 7 highlights key factors involved in neurogenesis,229–240 their impact on brain function, and their relevance to neurodegenerative diseases, alongside potential therapeutic implications.
Key molecular factors in neurogenesis, neurodegeneration, and their therapeutic potential
| Factor | Role in neurogenesis | Role in neurodegenerative diseases | Potential therapeutic implications | References |
|---|---|---|---|---|
| Tau | Tau stabilizes microtubules and regulates neuronal function, supporting neural progenitor differentiation, as shown in molecular and animal studies | Hyperphosphorylation of tau leads to neurofibrillary tangles, impairing neurogenesis and contributing to cognitive deficits, as demonstrated in molecular and human studies | Enhancing tau-related pathways may preserve neurogenesis and cognitive function | 229,230 |
| Amyloid-beta (Aβ) | Soluble Aβ enhances neurogenesis, whereas toxic Aβ oligomers suppress neural progenitor proliferation, as demonstrated in molecular and animal studies | Aβ accumulation induces synaptic dysfunction and neuronal loss, leading to cognitive decline in Alzheimer’s disease, as observed in animal and human studies | Reducing Aβ accumulation can improve neurogenesis and reverse AHN deficits | 231–233 |
| Presenilins | Presenilins regulate APP cleavage and Aβ peptide production, affecting neural progenitor cell survival, as shown in molecular and animal studies | Mutations in PS1 disrupt progenitor maintenance, impair synaptic plasticity, and contribute to neurodegeneration, as evidenced in molecular and human studies | Targeting presenilin-related pathways may restore neurogenesis | 234–236 |
| Apolipoprotein E (APOE) | APOE maintains neural progenitor pools and regulates hippocampal neurogenesis, as demonstrated in molecular and animal studies | The APOE ε4 allele is associated with impaired neurogenesis, increased Aβ deposition, and heightened Alzheimer’s disease risk, as shown in human molecular studies | Targeting APOE-related pathways may offer therapeutic benefits for AD | 237,238 |
| Alpha-synuclein | Alpha-synuclein influences dopamine metabolism and adult hippocampal neurogenesis, as observed in molecular and animal studies | Aggregation of α-synuclein impairs hippocampal neurogenesis and contributes to Parkinson’s disease pathology, as shown in animal models and human studies | Stimulating neurogenesis could counteract α-synuclein aggregation effects | 239,240 |
In the hippocampus, neurogenesis involves the production of neuroblasts, which either integrate into existing neural circuits or undergo apoptosis. This tightly regulated process is essential for maintaining the homeostasis and adaptability of the hippocampal network.24,108,241 Disruptions to this process, particularly with aging, compromise brain health and heighten vulnerability to neurodegenerative diseases.
As the brain ages, molecular and cellular changes accumulate, disrupting neurogenesis. Senescence markers such as p16, p19, p53, and HMGB1 impair the NSC pool,39,242 leading to diminished neurogenesis and cognitive decline. Understanding these molecular pathways is crucial for restoring neurogenesis and addressing the deficits observed in aging and neurodegenerative conditions.
Tau, a microtubule-associated protein essential for neuronal integrity, plays a significant role in neurogenesis. In AD, tau becomes pathologically hyperphosphorylated, leading to neurofibrillary tangles that disrupt neuronal function and impair neurogenesis.229,233,243,244 Experimental evidence suggests that reducing tau levels under stress conditions can enhance neurogenesis.
Aβ plays a dual role in neurogenesis. Soluble Aβ promotes neurogenesis, while aggregated Aβ exerts toxic effects, particularly in neurogenic regions like the dentate gyrus and SVZ.232,245 Strategies aimed at reducing Aβ aggregation have shown potential in restoring neurogenesis and improving cognitive function in preclinical studies, highlighting the complex role of Aβ in the brain.
Presenilins, components of the γ-secretase complex responsible for amyloid precursor protein cleavage, are essential for maintaining neural progenitor populations. Mutations in presenilin 1 reduce these populations and impair cognitive performance.246,247 Therapeutic interventions to restore presenilin function may rebalance neurogenesis and neurodegeneration in AD, presenting a promising avenue for treatment.
Apolipoprotein E (APOE) also plays a pivotal role in neurogenesis, particularly in regulating lipid metabolism and maintaining NSC pools.248,249 The APOE ε4 allele, a significant genetic risk factor for AD, is associated with reduced neurogenesis and accelerated cognitive decline.250 Targeting APOE-related pathways, especially in ε4 carriers, could help mitigate neurogenic deficits and provide cognitive protection.
In PD, the aggregation of alpha-synuclein disrupts neurogenesis, particularly in the dentate gyrus and olfactory bulb. Experimental models have shown that stimulating neurogenesis can counteract these disruptions,251–253 offering a potential therapeutic strategy to address PD-related deficits and improve cognitive and olfactory functions.
By elucidating the molecular mechanisms governing neurogenesis and their disruption in aging and neurodegenerative diseases, researchers can identify novel therapeutic targets. Enhancing neurogenesis offers significant promise for counteracting cognitive decline and neural impairments associated with these conditions, paving the way for innovative interventions and treatments.
Neurogenic decline in the aging SVZ: Cellular, molecular, microenvironmental challenges, and strategies for rejuvenation
Aging is strongly associated with a significant decline in neurogenesis within the SVZ, one of the primary neurogenic niches in the adult brain.254 The SVZ plays a critical role in generating new neurons, primarily destined for the olfactory bulb. However, aging disrupts this process, leading to reduced proliferative activity and an increased proportion of quiescent NSCs.42 This age-related shift in the balance between active and quiescent NSCs diminishes the regenerative capacity of the SVZ, contributing to cognitive decline and impaired recovery following brain injuries.
The cellular and molecular changes in the aging SVZ are multifaceted (Table 8).39,127,174,186,254–260 These changes include a reduced number of transit-amplifying cells (TACs), lower proliferation rates, and decreased neuroblast production, which collectively result in a marked decline in neurogenic output. Consequently, the functional contributions of SVZ-derived neurons are significantly compromised. This decline in SVZ neurogenesis with age has profound implications for brain health,39 as the diminished ability to generate new neurons limits the brain’s capacity to adapt to injury and maintain cognitive function.
Age-related cellular changes in the subventricular zone and their impact on neurogenesis
| Cell type | Change with aging | Impact | References |
|---|---|---|---|
| Quiescent NSCs (qNSCs) | Molecular and animal studies indicate an increased proportion of quiescent NSCs, reflecting a shift from an active to a quiescent state with aging | The rise in quiescent NSCs reduces the pool of available stem cells for activation and differentiation, leading to a decline in neurogenic capacity | 174,186,255,256 |
| Active NSCs (aNSCs) | Animal studies demonstrate a progressive decline in active NSC numbers, reducing the frequency of cell cycle re-entry | The depletion of active NSCs limits neurogenesis, contributing to age-related cognitive impairment | 39,127,254 |
| Transit-Amplifying Cells (TACs) | Molecular and animal studies report reduced proliferation of TACs, leading to a decline in progenitor cell transition from NSCs | A reduction in TAC expansion compromises progenitor cell populations, ultimately decreasing neuroblast and neuron production | 257,258 |
| Neuroblasts (NBs) | Animal and human studies reveal a decline in neuroblast populations, indicating impaired differentiation and migration toward the olfactory bulb | Fewer neuroblasts reduce the incorporation of new neurons into the olfactory bulb, weakening sensory processing and cognitive adaptability | 259,260 |
Age-related microenvironmental alterations in the subventricular zone and their impact on neurogenesis
The neurogenic microenvironment of the SVZ undergoes significant age-related changes that disrupt its structural, cellular, and molecular integrity, leading to a decline in neurogenesis. These alterations impair NSC function, diminishing the brain’s regenerative capacity.261,262 Key changes include microglial activation, ependymal layer degeneration, and vascular decline, each contributing uniquely to reduced neurogenic potential (Table 9).263–271
Key microenvironmental changes in the aging subventricular zone and their impact on neurogenesis
| Feature | Age-related change | Consequences | References |
|---|---|---|---|
| Microglia | Animal and molecular studies indicate that aging leads to increased release of pro-inflammatory cytokines by microglia | This heightened inflammatory state contributes to NSC quiescence, thereby reducing their activation and differentiation potential | 263–266 |
| Ependymal Layer | Findings from both animal and human studies reveal that aging causes degeneration of the ependymal layer, accompanied by astrocytic and microglial infiltration | This structural deterioration disrupts the NSC-cerebrospinal fluid (CSF) interface, impairing NSC signaling and function | 267,268 |
| Vasculature | Human and animal research demonstrates that aging is associated with a decline in vascular integrity, characterized by reduced vascular density and branching complexity | These vascular changes impair blood flow, oxygenation, and nutrient delivery to NSCs, ultimately restricting their proliferation and neurogenic activity | 269–271 |
Microglial activation is a prominent feature of aging in the SVZ. With age, microglia adopt a pro-inflammatory phenotype, characterized by increased production of pro-inflammatory cytokines.272,273 This heightened inflammatory response suppresses NSC proliferation and differentiation by inducing quiescence.274 Consequently, the activation and regenerative capacity of NSCs are significantly reduced, contributing to the observed decline in neurogenesis in the aging brain.
The degeneration of the ependymal layer, which serves as a critical interface between CSF and NSCs, further exacerbates neurogenic decline. Aging thins the ependymal layer, allowing infiltration by astrocytes and microglia, which disrupts the signaling mechanisms necessary for NSC activation and maintenance.186,267,275 This breakdown in the NSC-CSF interface compromises NSC functionality and reduces the neurogenic output of the SVZ.
Vascular decline is another hallmark of the aging SVZ, marked by reduced vascular density and branching complexity. These changes restrict blood flow, limiting the delivery of oxygen and nutrients essential for NSC maintenance and regeneration.276,277 The resulting metabolic insufficiency further impairs NSC function, compounding the decline in neurogenesis.
Together, these structural, cellular, and molecular alterations create a hostile microenvironment for NSCs, significantly impairing their ability to maintain neurogenic activity in the aging brain. The pro-inflammatory state induced by microglial activation, the structural deterioration of the ependymal layer, and the decline in vascular support collectively limit the SVZ’s capacity for neurogenesis.
Molecular and transcriptomic alterations in the aging subventricular zone impair neurogenesis
Aging induces a cascade of molecular changes within the SVZ, compounding structural alterations to further impair neurogenesis. These changes include disrupted gene expression, increased cellular heterogeneity, and heightened inflammation,131,278 all of which contribute to the decline in the brain’s regenerative capacity.
A notable molecular alteration is the downregulation of genes critical for the proliferation of TACs and the generation of neuroblasts. This decline in gene expression significantly hampers NSC activity, limiting the production of new neurons and diminishing the neurogenic potential of the SVZ.11,105,279,280 Despite attempts by some TAC subpopulations to compensate through increased proliferation, this response is insufficient to sustain long-term neurogenesis, ultimately contributing to the progressive decline in regeneration.
Cellular heterogeneity in the aging SVZ further complicates the neurogenic landscape. The presence of subpopulations attempting compensatory activity contrasts with the quiescence of others, creating an imbalance that undermines the overall regenerative capacity of the region.186,281,282
Inflammation is another critical factor in the aging SVZ, with progenitor cells and microglia displaying markers of chronic, low-grade inflammation.42,186,190 This persistent inflammatory state promotes NSC quiescence, reducing their activation, proliferation, and differentiation capabilities. The resultant decline in neurogenesis exacerbates the aging brain’s vulnerability to cognitive decline and injury.
Additionally, recent studies highlight the infiltration of clonally expanded T-cells into the aging SVZ.42,283,284 These immune cells secrete interferon-γ, intensifying the inflammatory milieu and further suppressing neurogenic activity. The presence of these activated T-cells disrupts the neurogenic niche, placing additional strain on NSC function.
These molecular and transcriptomic changes create an increasingly adverse environment for NSCs. The cumulative effects of downregulated gene expression, cellular heterogeneity, chronic inflammation, and immune cell infiltration significantly impair neurogenesis, highlighting the complexity of age-related decline in the SVZ.
Strategies to rejuvenate neurogenesis in the aging subventricular zone
Efforts to restore neurogenesis in the aging SVZ focus on systemic and niche-specific approaches to enhance the regenerative potential of NSCs. These strategies aim to improve the surrounding microenvironment or directly modulate biological processes that are crucial for NSC function (Table 10).42,186,285–292
Strategies and mechanisms for enhancing neurogenesis in the aging subventricular zone
| Intervention | Mechanism | Example agents | References |
|---|---|---|---|
| Circulating Factors | Rejuvenates the systemic environment and improves NSC function, as demonstrated in human and molecular studies | GDF11, TIMP2 | 186,285 |
| Anti-Aging Molecules | Modulates pro-aging molecules to counteract age-related changes, as shown in animal and molecular studies | CCL11, TGF-β | 42,186,286 |
| Dietary Interventions | Enhances neurogenesis through metabolic changes, as evidenced in human, animal, and molecular studies | Caloric restriction, FMD | 287–289 |
| Pharmacological Agents | Promotes NSC activity, reduces inflammation, and supports autophagy, as demonstrated in animal studies and pharmacological investigations | Rapamycin, Metformin | 290–292 |
Heterochronic parabiosis, which involves exposing aged animals to youthful blood circulation, has shown remarkable potential in rejuvenating neurogenesis by improving systemic factors.293,294 Molecules such as growth differentiation factor 11 and tissue inhibitor of metalloproteinases 2 have been identified as youth-promoting agents that enhance NSC function.295,296 Conversely, reducing pro-aging factors such as chemokine ligand 11 and transforming growth factor-beta has demonstrated the potential to counteract age-related declines in neurogenesis,96,297,298 highlighting the importance of systemic factors in maintaining neurogenic capacity.
Dietary and pharmacological interventions have emerged as promising strategies to mitigate age-related neurogenic decline. Caloric restriction (CR) and fasting-mimicking diets have been shown to preserve neuroblast populations and promote neurogenesis in the olfactory bulb,42,287,299 offering insights into dietary modulation as a tool for supporting brain health. Pharmacological agents such as rapamycin and metformin target specific cellular pathways, including autophagy and insulin-like growth factor 1 signaling, to promote NSC activity, reduce quiescence, and enhance neurogenic capacity in the aging SVZ.300–302
Chronic inflammation and cellular senescence are significant contributors to the decline in neurogenesis. Anti-inflammatory therapies, such as blocking cytokines like TNF-α, have shown promise in restoring NSC function and enhancing neurogenesis.295,303,304 Furthermore, senolytic agents like Dasatinib and Quercetin selectively eliminate senescent cells, alleviating their adverse impact on the SVZ microenvironment and rescuing neurogenic activity.283,305,306
By addressing chronic inflammation, cellular senescence, and age-related molecular changes, these interventions offer promising avenues for enhancing neurogenesis and combating cognitive decline and neurodegenerative diseases associated with aging.
Pharmacological and metabolic interventions targeting aging, neurogenesis, and neurodegeneration: Mechanisms and therapeutic potential
Pharmacological interventions for aging and neurodegeneration: Neurogenesis, protection, and lifespan extension
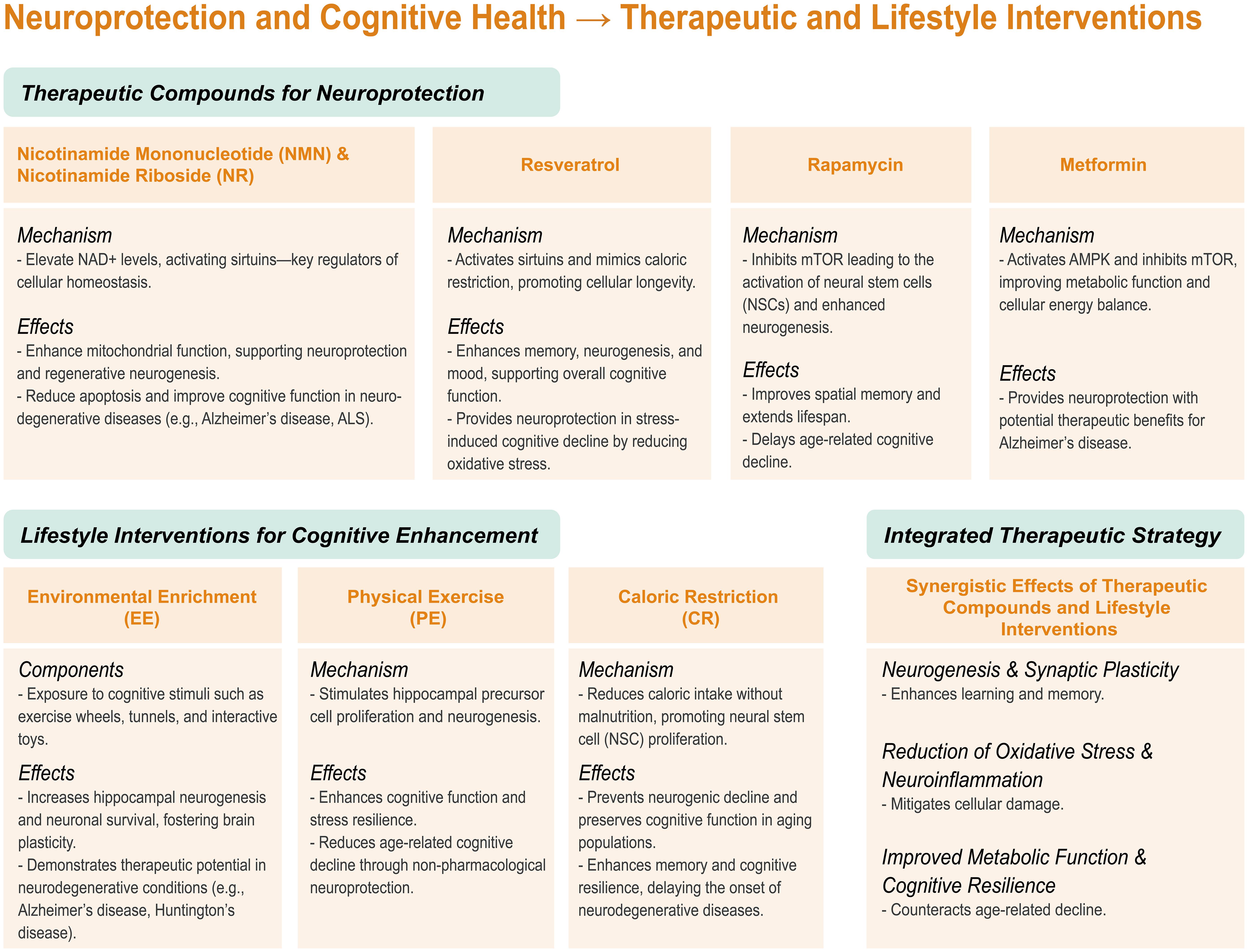
Pharmacological compounds targeting aging and neurodegeneration can be classified into three primary categories: those that stimulate neurogenesis, protect against neurodegeneration, and extend lifespan (Table 11).307–324 Understanding how compounds intersect these categories—including NAD+-boosting molecules, resveratrol, rapamycin, and metformin—is essential for targeting aging and neurodegeneration. These agents have been widely studied for their roles in aging, neurogenesis, and neurodegenerative diseases, primarily through the modulation of metabolic pathways.
Therapeutic compounds for neurogenesis and aging: Targeted metabolic pathways and their effects on cognitive and neurological health
| Compound | Category | Metabolic pathway targeted | Primary effects | Research focus | References |
|---|---|---|---|---|---|
| Nicotinamide Mononucleotide (NMN) | Neurogenesis, Neuroprotection | NAD+ precursor, Sirtuin activation | Improves cognitive function, reduces apoptosis, promotes regenerative neurogenesis in ischemic conditions | Aging, cognitive decline, neurodegenerative diseases (e.g., AD, ALS) | 307–310 |
| Nicotinamide Riboside (NR) | Neurogenesis, Neuroprotection | NAD+ precursor, Sirtuin activation | Enhances neurogenesis, improves cognitive function, reduces neuroinflammation | Aging, neurodegeneration (e.g., AD, ALS) | 311–314 |
| Resveratrol | Neurogenesis, Neuroprotection | Sirtuin activation, mimicry of caloric restriction | Enhances memory, neurogenesis, improves mood, mitigates cognitive deficits | Aging, cognitive decline, neuroprotection, stress-induced impairments | 315–317 |
| Rapamycin | Neurogenesis, Lifespan extension | mTOR inhibition | Improves spatial memory, cognitive function, NSC activation, extends lifespan | Aging, neurodegeneration, lifespan extension, cognitive function | 318–321 |
| Metformin | Neuroprotection, Lifespan extension | AMPK activation, mTOR inhibition | Improves metabolic health, prevents age-related diseases, extends healthspan | Aging, diabetes, metabolic health, neurodegenerative diseases | 322–324 |
Nicotinamide mononucleotide (NMN), a precursor to NAD+, has garnered attention for its potential to restore cellular processes impaired by age-related NAD+ depletion. In preclinical studies, NMN has shown improvements in cognitive function, reductions in apoptosis, and promotion of regenerative neurogenesis under ischemic conditions (Fig. 2).307,325 However, NMN has shown limited efficacy in enhancing NSC proliferation in aged models, suggesting a potential age-related limitation. Similarly, nicotinamide riboside (NR), another NAD+ precursor, enhances sirtuin activation and demonstrates significant neuroprotective effects. NR has been shown to improve cognitive function, reduce neuroinflammation, and support NSC survival and proliferation, particularly in AD and amyotrophic lateral sclerosis models.308,311,326 While NR exhibits mild lifespan-extending properties, its translation into human applications requires further validation.
Resveratrol, a polyphenolic compound, activates sirtuins and mimics the effects of caloric restriction, making it a promising candidate for aging and neurodegenerative conditions. Preclinical studies indicate that resveratrol improves hippocampal neurogenesis, enhances memory, and mitigates cognitive deficits induced by hypoxia or chronic stress.315,327 However, clinical trials have yielded mixed results, with some showing cognitive improvements in older adults and others revealing negligible effects. This inconsistency underscores the need for additional research to better understand its therapeutic potential.
Rapamycin, an inhibitor of the mechanistic target of rapamycin, has shown promise in aging and neurogenesis research. Studies have demonstrated its ability to enhance spatial learning, memory, and NSC activation in aging models.39,318,319 However, its effects on hippocampal neurogenesis remain inconsistent, with some studies showing positive outcomes and others reporting minimal impact. Short-term clinical trials suggest that rapamycin may improve cognitive function, but long-term studies are needed to confirm its efficacy in treating age-related cognitive impairments.
Metformin, a widely used anti-diabetic drug, activates AMP-activated protein kinase and exerts caloric restriction-like effects. Preclinical studies have highlighted metformin’s potential to improve healthspan, reduce the risk of age-related diseases, and provide neuroprotective effects.323,324,328 Ongoing large-scale clinical trials, such as MILES and TAME, aim to evaluate its anti-aging properties and its ability to prevent neurodegenerative diseases.
The impact of physical and metabolic interventions on neurogenesis and cognitive function
Several physical and metabolic interventions, including physical exercise, environmental enrichment, and CR, have demonstrated the ability to enhance neurogenesis and are linked to extended lifespan and improved healthspan across various model organisms (Table 12).329–338 Although these interventions operate through distinct mechanisms, they offer complementary effects on neurogenesis and cognitive function, each targeting unique aspects of brain health. Together, they represent promising strategies for improving brain function and mitigating age-related cognitive decline.
Impact of brain health interventions on neurogenesis, cognitive function, and therapeutic potential
| Intervention | Mechanism of action | Impact on neurogenesis | Cognitive function impact | Therapeutic potential | References |
|---|---|---|---|---|---|
| Environmental Enrichment (EE) | Stimulating environment with interactive objects (wheels, tunnels, toys) | Increases neurogenesis in the hippocampal DG; enhances survival of new neurons | Improves learning, memory, and cognitive function | Potential therapeutic role in AD and HD models | 329–331 |
| Physical Exercise (PE) | Voluntary running or treadmill activity | Stimulates hippocampal precursor cell proliferation | Mitigates cognitive deficits due to stress/injury | Improves cognition in Alzheimer’s models; systemic benefits on brain health | 332–334 |
| Caloric Restriction (CR) | Reduction in caloric intake without malnutrition | Increases NSC proliferation and prevents neurogenic decline | Improves memory, particularly in aging populations | Potential for improving cognitive function in older adults, with ongoing research in humans | 335–338 |
Physical exercise, such as voluntary running or treadmill activity, stimulates the proliferation of neural precursor cells, particularly in the hippocampus, enhancing neurogenesis and promoting cognitive function.208,339,340 This occurs through mechanisms such as neuroplasticity, inflammation reduction, and increased neurotrophic factor levels. Additionally, exercise provides systemic benefits, as studies show that plasma from exercised animals can boost neurogenesis and cognitive function in sedentary individuals. Environmental enrichment, by contrast, involves providing a stimulating environment that promotes learning, memory, and overall brain health by supporting the survival of newly generated neurons in the hippocampus.329,341,342 Environmental enrichment has therapeutic potential in neurodegenerative diseases like Alzheimer’s and Huntington’s disease. Finally, CR, which reduces caloric intake without malnutrition, promotes neural stem cell proliferation and counters age-related neurogenic decline, particularly in the hippocampus and subventricular zone.186,343–345 This results in improved memory and cognitive function, especially in aging animals, and contrasts with high-fat diets, which hinder neurogenesis. Although most CR research has been conducted in animal models, human studies suggest it may enhance memory function, particularly in older adults or those with obesity. However, further research is needed to fully understand its effects on hippocampal function and aging in humans. Moreover, challenges arise when attempting to apply these interventions in human clinical settings, as the long-term sustainability of CR and exercise regimens may be difficult to maintain, and adherence to environmental enrichment protocols can be challenging in human populations. As with pharmacological interventions, translating the beneficial effects of physical and metabolic interventions into human therapies requires a deeper understanding of individual variability, the impact of comorbidities, and the complex interactions between lifestyle factors and brain health.
While pharmacological and metabolic interventions offer significant therapeutic potential for aging, neurogenesis, and neurodegeneration, further studies are required to overcome the challenges of translating findings from animal models to human therapies. The mechanisms underlying these interventions, though promising, must be thoroughly investigated to optimize their clinical applicability and ensure their safety and efficacy in human populations.
Discussion, research gaps, and future directions
Adult neurogenesis is crucial for maintaining cognitive function, promoting brain plasticity, and enhancing resilience to age-related cognitive decline. Disruptions in neurogenic pathways—caused by factors such as hippocampal dysfunction, oxidative stress, cannabinoid signaling dysregulation, neuroinflammation, and metabolic disturbances—contribute to cognitive decline with age. While adult neurogenesis has been well-documented in rodent models, its persistence and significance in humans, particularly older adults, remain contentious. These discrepancies likely stem from variations in detection methods, tissue processing, and sample preservation. The development of advanced imaging technologies and single-cell genomics offers promising opportunities to resolve these uncertainties, providing deeper insights into neurogenesis in the context of brain aging, cognitive decline, and neurodegenerative diseases.
Despite significant progress in understanding adult neurogenesis, several critical knowledge gaps persist. A more comprehensive exploration of the signaling pathways, growth factor interactions, and epigenetic modifications regulating neurogenesis—particularly in aging—is essential. Current research has yet to fully elucidate how these factors interact to contribute to age-related neurogenic decline. Potential interventions to restore neurogenic capacity include enhancing DNA demethylase activity, improving vascular support, optimizing mitochondrial function, and modulating hormonal balance. However, translating these strategies into clinical therapies presents challenges, including species-specific differences, genetic variability, and environmental influences.
One promising therapeutic approach is reactivating dormant NSCs and TAPs to combat neurodegenerative diseases and cognitive decline. However, this strategy carries risks, such as aberrant neurogenesis, tumor formation, and long-term safety concerns. Developing multifaceted therapies that combine growth factor modulation, mitochondrial restoration, and hormonal regulation may offer a more comprehensive solution to neurogenic decline during aging.
Pathological factors, such as tau, Aβ, presenilins, APOE, and alpha-synuclein, significantly impair neurogenesis and drive neurodegenerative disease progression. Tau hyperphosphorylation destabilizes neuronal structures, while toxic Aβ aggregates inhibit neurogenesis, exacerbating AD pathology. Genetic mutations in presenilins, the APOE ε4 allele, and alpha-synuclein aggregation further diminish neurogenic potential in AD and PD. Targeting these molecular mechanisms could provide innovative therapeutic strategies aimed at restoring neurogenesis in these diseases.
Environmental and lifestyle factors, such as physical exercise, caloric restriction, and environmental enrichment, have demonstrated potential in enhancing neurogenesis and improving cognitive resilience. Pharmacological agents like NMN, NR, resveratrol, rapamycin, and metformin also show promise in preclinical models, though their clinical translation is hindered by inconsistent efficacy and a lack of robust clinical trials. Further research is needed to identify reliable biomarkers and optimize these interventions for clinical use.
Despite these advancements, significant research gaps persist in the study of adult neurogenesis. Translational challenges between rodent models and human studies remain, due to species-specific differences and the complexities of the human brain. Additionally, long-term studies are essential to evaluate the safety, efficacy, and potential tumorigenic risks associated with NSC-based therapies. While promising preclinical findings exist, integrating these therapies into clinical practice requires further investigation to better understand the underlying molecular mechanisms and assess long-term outcomes.
A critical area of research is the epigenetic and molecular pathways involved in age-related neurogenic decline. Understanding how these pathways influence neurogenesis across the lifespan could uncover new targets for intervention. Further studies are also needed to evaluate the effectiveness of environmental, lifestyle, and pharmacological interventions in promoting neurogenesis and mitigating cognitive decline. Developing biomarkers to reliably measure neurogenic activity in humans will be crucial for optimizing therapeutic strategies and tracking progress in clinical trials. Ethical considerations surrounding gene expression modifications and cellular reprogramming techniques must also be addressed to ensure the safety and equity of clinical applications.
One critical next step in research is the development of non-invasive, high-resolution imaging methods to study neurogenesis in living humans. Advances in in vivo imaging technologies, such as magnetic resonance imaging and positron emission tomography, hold the potential to provide real-time insights into neurogenesis and its role in aging and neurodegeneration. These innovations could allow direct observation of neurogenic processes in human subjects, enhancing our understanding of brain aging.
Another key area for future research is identifying reliable biomarkers to measure neurogenesis, particularly in patients with AD. These biomarkers are crucial for tracking disease progression and evaluating the effectiveness of therapeutic interventions. Techniques such as single-cell RNA sequencing and imaging-based assays targeting specific neurogenic processes hold promise for identifying biomarkers that could be used in clinical settings.
Furthermore, future research should focus on identifying and testing safe and effective methods to reactivate dormant NSCs and TAPs without triggering abnormal brain activity or tumor formation. Strategies such as localized gene editing, small molecule modulators, and optimizing growth factor signaling could be explored. Reactivating NSCs holds significant potential for treating neurodegenerative diseases and combating cognitive decline, but ensuring safety and long-term efficacy will be paramount.
Conclusions
Significant progress has been made in the field of adult neurogenesis, yet a comprehensive understanding of its role in aging and neurodegeneration remains elusive. Addressing the research gaps highlighted in this review—particularly those concerning the molecular and epigenetic mechanisms that regulate neurogenesis and the translation of preclinical findings into clinical practice—will be essential. Bridging these gaps could lead to the development of innovative therapies that preserve or restore neurogenesis, mitigate cognitive decline, enhance resilience to neurodegenerative diseases, and ultimately improve the quality of life for aging populations.
Declarations
Acknowledgement
None.
Funding
This research received no external funding.
Conflict of interest
The authors declare no conflict of interest.
Authors’ contributions
NMM is the sole author of the manuscript.

 Author information
Author information