Introduction
Disruption of the intestinal barrier is a hallmark pathological feature of chronic inflammatory bowel diseases (IBDs), with its most prominent manifestations observed in IBD, specifically in its two clinical subtypes: Crohn’s disease (CD) and ulcerative colitis (UC).1 Autophagy is a vital self-degradative recycling mechanism that maintains cellular homeostasis by clearing damaged components and recycling macromolecules.2 Studies have shown that autophagy in intestinal epithelial cells (IECs) protects the intestinal barrier by removing intracellular pathogens and modulating inflammatory responses, thereby reducing IBD symptoms.3 Moreover, numerous studies have examined the interaction between autophagy and three fundamental biological pathways in IBD: NOD-like receptor protein 3 (NLRP3) inflammasome activation, endoplasmic reticulum stress (ERS), and reactive oxygen species (ROS) production. ROS also plays a key role in NLRP3 inflammasome-mediated inflammatory responses. Based on this, we propose the concept of the ROX/NLRP3 axis. Activation of the NLRP3 inflammasome leads to the release of intracellular inflammatory cytoses, exacerbating gut inflammation.4,5 Additionally, ERS worsens the pathological state of IBD by impairing IEC function,6,7 while excessive ROS production not only damages intestinal cells but also induces inflammatory responses, further aggravating IBD. Dysregulation of autophagy in IECs initiates a pathogenic cascade, wherein IEC dysfunction compromises intestinal barrier integrity. This breach concurrently activates ERS and amplifies ROS generation, which, in turn, propagates NLRP3 inflammasome activation through redox-sensitive signaling pathways. The convergence of these interlinked mechanisms—barrier dysfunction, organelle stress, oxidative bursts, and inflammatory priming—establishes a self-perpetuating loop that fundamentally drives UC pathogenesis.8 Therefore, this review aimed to explore the relationship between autophagy and these mechanisms in greater depth to enhance our understanding of IBD pathogenesis and identify novel therapeutic targets.
Overview of autophagy in IBD pathogenesis
Autophagy operates as a cytoplasmic nutrient salvage pathway, executing critical nutrient recycling under metabolic stress to maintain cellular energy homeostasis.9 It also degrades specific cytoplasmic components, such as damaged mitochondria through mitophagy or intracellular bacteria via xenophagy.10,11 Autophagosomes eventually fuse with lysosomes, where their contents are degraded by lysosomal enzymes, while ROS oxidize and neutralize certain molecules and pathogens through oxidation.12 The process of autophagy involves the regulation of several key proteins and signaling pathways, such as the mammalian target of rapamycin (mTOR) signaling pathway, the unc-51 like autophagy activating kinase 1 complex, and the transport of light chain 3 proteins, which work together to ensure that cells maintain homeostasis in response to nutrient deficiencies, oxidative stress, and other stressors.13 Accumulating evidence demonstrates that autophagy exerts a cytoprotective function in IBD pathogenesis. In IBD patients, autophagic dysfunction compromises intestinal epithelial cell-mediated bacterial clearance, thereby potentiating sustained mucosal inflammation.14 Enhancing autophagic function to improve intestinal inflammation and epithelial barrier function15 may offer a novel strategy for the treatment of IBD.
Autophagy with ROS/NLRP3 axis
In the previous discussion, we identified the ROS-NLRP3 inflammasome axis and its downstream signaling cascades as critical drivers of intestinal inflammatory activation. We further explore the mechanistic contributions of this axis to IBD pathogenesis while systematically evaluating autophagy’s homeostatic regulation of redox-inflammasome crosstalk.
ROS in NLRP3 activation
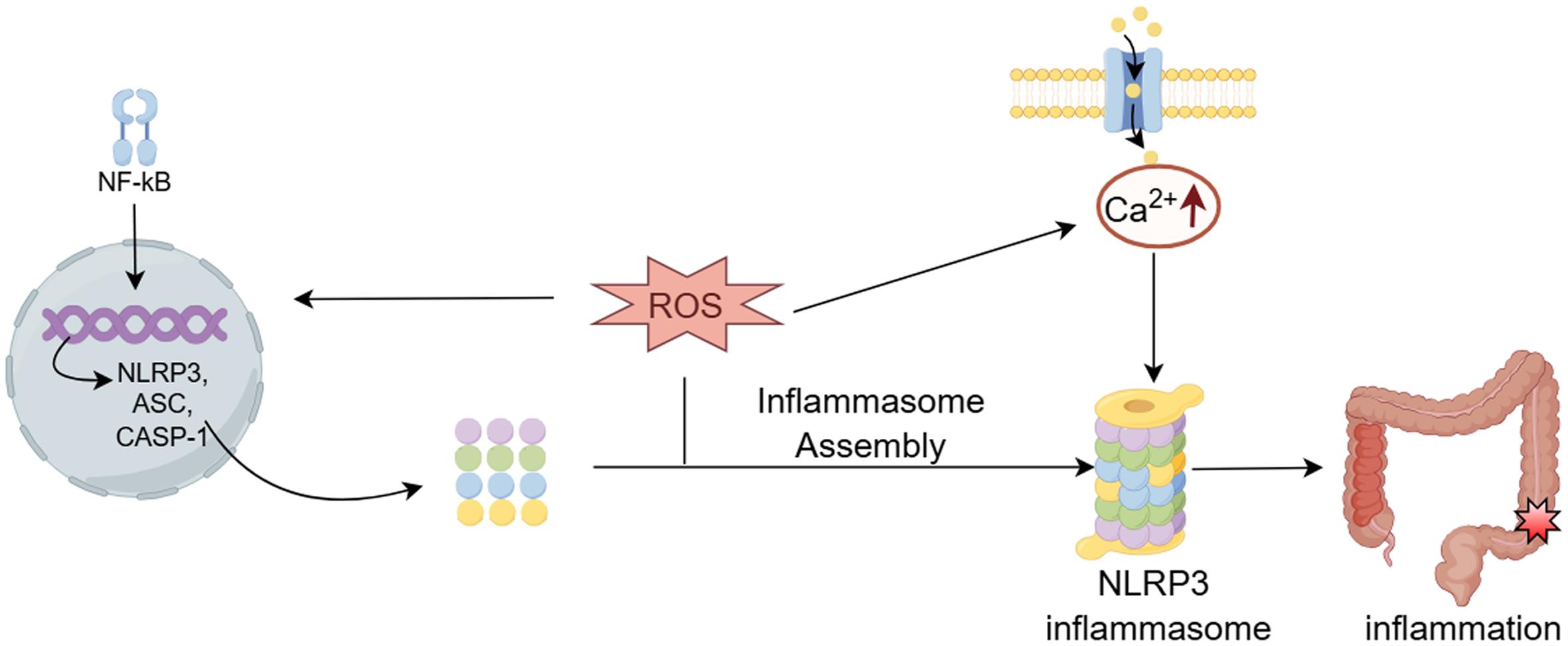
ROS act as critical mediators of NLRP3 inflammasome activation, driving both the structural assembly of NLRP3, apoptosis-associated speck-like protein containing CARD (ASC), and pro-caspase-1 into functional inflammasome complexes, as well as the transcriptional amplification of NLRP3, caspase-1, and associated pro-inflammatory cytokines through redox-sensitive signaling cascades (Fig. 1).16 NADPH oxidase (NOX)-generated ROS are essential mediators of NLRP3 inflammasome-dependent caspase-1 activation and interleukin (IL)-1β maturation. Experimental evidence from murine models demonstrates that NOX2-derived oxidative signaling is indispensable for glomerular NLRP3 inflammasome activation following podocyte injury.17 NOX-derived ROS are implicated in the priming phase of NLRP3 inflammasome activation, where NOX4-generated ROS critically regulate nuclear factor-κB (NF-κB)-dependent transcriptional programming to upregulate NLRP3 expression and pro-IL-1β synthesis, establishing the molecular prerequisites for inflammasome assembly.18 Additionally, ROS production is closely linked to Ca2+ influx, which has been identified as a key mechanism for NLRP3 inflammasome activation. Notably, the Ca2+-permeable channel transient receptor potential melastatin-2 acts as a specific activator of NLRP3 under oxidative stress conditions.19 Knockout or inhibition of transient receptor potential melastatin-2 abolishes ROS-dependent NLRP3 inflammasome activation in macrophages and monocytes.20,21 Experimental evidence indicates that targeted inhibition of mitochondrial ROS generation attenuates NLRP3 inflammasome activation by suppressing ASC speck formation, thereby mitigating caspase-1-mediated pyroptosis and IL-1β-driven inflammatory pathology.22 This suggests a potential new approach for the treatment of NLRP3-related diseases.
ROS may induce intracellular changes through oxidative stress, nuclear factor-κB (NF-κB) activation, and upregulation of inflammasome components (NLRP3, caspase-1, and apoptosis-associated speck-like protein containing CARD (ASC)), promoting NLRP3 inflammasome assembly. Additionally, ROS generation is closely related to calcium ion influx, an upstream signal of NLRP3 activation, and is considered an important mechanism of NLRP3 activation. CASP-1, Cysteine-aspartic acid protease-1. (This figure was created by Figdraw).
ROS/NLRP3 axis in the release of inflammatory mediators
The ROS/NLRP3 signaling axis orchestrates the release of inflammatory mediators through caspase-1-dependent maturation of pro-IL-1β and IL-18 via NLRP3 inflammasome activation. These cytokines amplify inflammatory cascades through autocrine/paracrine activation of NF-κB and signal transducer and activator of transcription 3 signaling pathways. Studies have shown that ROS can exacerbate inflammatory responses by activating the NLRP3 inflammasome and promoting the release of these inflammatory cytokines.23 For example, lipopolysaccharide stimulates the inflammatory response of microglia through the ROS/NLRP3 axis.24 Therefore, therapeutic modulation of ROS generation and NLRP3 inflammasome activation emerges as a promising dual-target strategy to suppress pro-inflammatory mediator release and attenuate maladaptive immune responses in inflammatory pathologies.
ROS/NLRP3 axis in IBD
As an autoimmune disease, IBD involves the ROS/NLRP3 axis, which plays an important role in its pathological progression. For example, the ROS-NLRP3 inflammasome-IL-1β axis may contribute to platelet hyperactivity in active CD, potentially increasing the risk of intestinal micro-thrombosis. This study uncovered that platelets isolated from patients with active Crohn’s disease exhibited significantly elevated intracellular ROS levels, accompanied by upregulated expression of ASC, NLRP3 inflammasome components, and activated caspase-1, compared to healthy donors. These factors were positively correlated with platelet P-selectin exposure and fibrinogen binding.25 Further studies revealed that diallyl trisulfide has the potential to prevent and treat UC by inhibiting NLRP3 inflammasome activation via the Trx-1/ROS pathway. Diallyl trisulfide treatment notably reduced the levels of IL-1β and IL-18 and inhibited NLRP3 inflammasome activation by suppressing NF-κB pathway activation and the expression of Trx-1 in the colon.26In vitro and murine model analyses demonstrated that dimethyl fumarate and its bioactive intestinal metabolites suppress NLRP3 inflammasome activation through Nrf2-dependent attenuation of mitochondrial oxidative stress, involving reduced ROS-mediated oxidation of mitochondrial DNA and consequent inhibition of caspase-1 proteolytic cleavage via impaired ASC oligomerization.27
The ROS/NLRP3 axis is also involved in the regulation of intestinal immune cells. A study found that Atox1 regulates macrophage polarization in intestinal inflammation via the ROS-NLRP3 inflammasome pathway. Atox1 drives M1-skewed macrophage polarization and amplifies the burden of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) in the intestinal mucosa through redox-sensitive potentiation of NLRP3 inflammasome assembly via mitochondrial ROS-mediated ASC oligomerization.28
Regulatory role of autophagy in the ROS/NLRP3 axis
Autophagy is not only an important pathway for cells to remove damaged components but also plays a key role in regulating the ROS/NLRP3 axis. Studies have found that autophagy can reduce the inflammatory response triggered by ROS by scavenging excess ROS and inhibiting NLRP3 inflammasome activation. For example, certain natural products, such as sulforaphane, have been shown to protect nerve cells from damage by downregulating ROS levels and enhancing autophagic activity through inhibition of the NLRP3 signaling pathway in microglia.29 Additionally, autophagy can play a protective role in various pathological states by promoting the degradation of NLRP3 inflammasome components and inhibiting the release of intracellular inflammatory factors.30,31 Therefore, autophagy plays a dual regulatory role in the ROS/NLRP3 axis, which can not only inhibit NLRP3 activation by clearing ROS, but also affect the inflammatory state of cells by regulating autophagy levels. This provides new ideas and targets for the treatment of related diseases.
A deeper understanding of the role of the ROS/NLRP3 axis in autophagy and inflammation can reveal the complex pathological mechanisms of IBD and provide a theoretical basis for developing novel therapeutic strategies. Modulating this axis may lead to innovative treatment options for the clinical management of IBD, such as antioxidants or drugs that specifically inhibit NLRP3 to reduce intestinal inflammation and promote autophagy, thereby improving patient outcomes.
Process of autophagy in intestinal epithelial cells
Intestinal epithelial autophagy
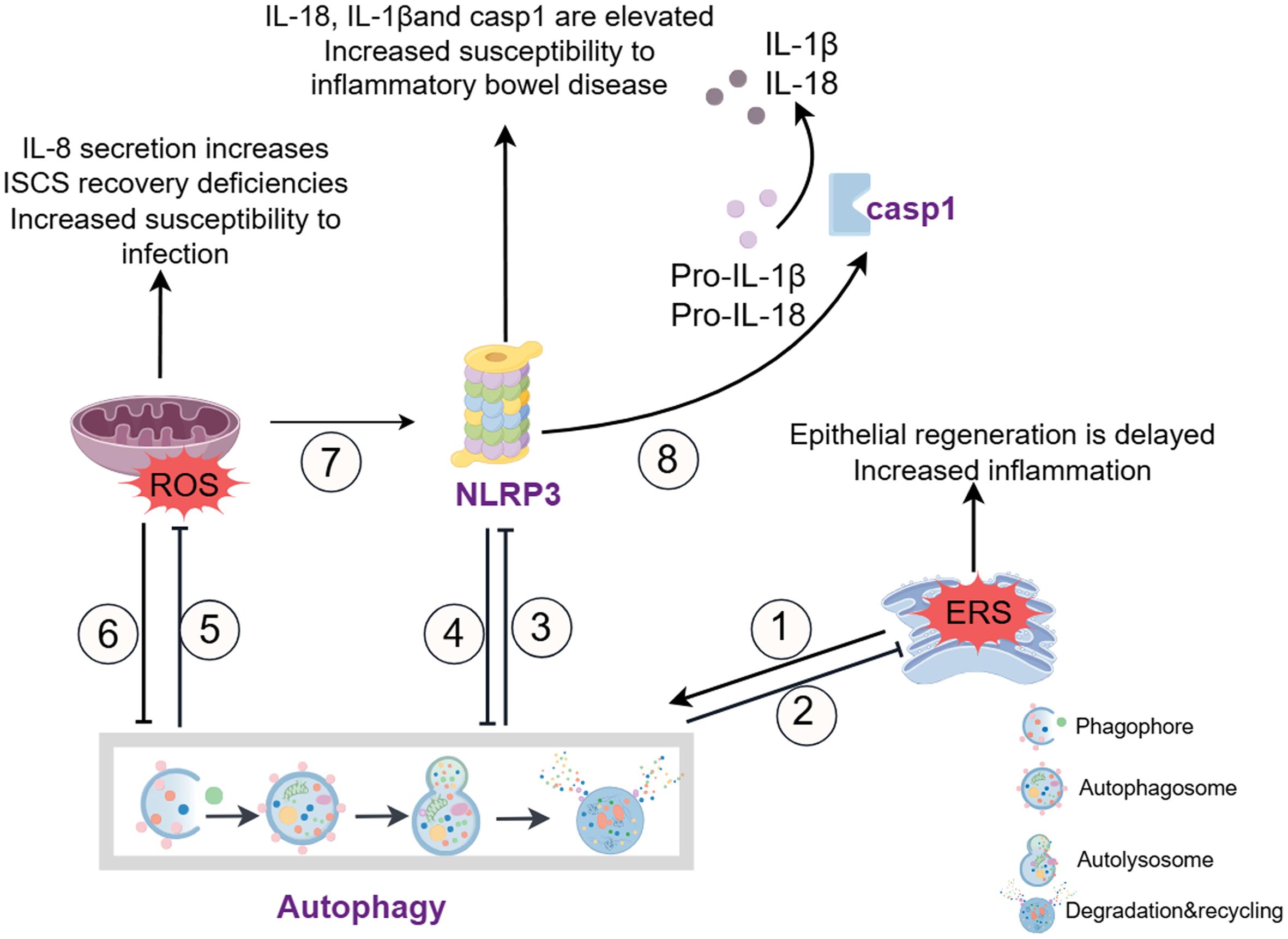
The regulation of autophagy in IECS includes the complex interplay of multiple signaling pathways (Fig. 2). For example, short-chain fatty acids alleviate colitis symptoms by stabilizing hypoxia-inducible factor 1α, which induces autophagy in intestinal epithelial cells.14 Lactic acid bacteria induce autophagy through the myeloid differentiation factor 88 pathway, enhancing the integrity of tight junctions and promoting the barrier function of intestinal epithelial cells.32 In addition to IECs, autophagy plays a critical role in immune cells such as macrophages and dendritic cells, which are essential for maintaining intestinal immune homeostasis. For instance, NOX4-dependent ROS production has been shown to mediate inflammatory macrophages, promoting damage to the intestinal mucosal barrier.22 Autophagy in macrophages regulates the clearance of intracellular pathogens and the release of pro-inflammatory cytokines, influencing the severity of IBD.22 Furthermore, dysfunctional autophagy in dendritic cells has been linked to impaired antigen presentation and T cell activation, contributing to chronic inflammation in IBD.18 The interaction of Polo-like kinase 1 and the mTOR axis has been found to modulate autophagy, preventing impairment of intestinal barrier function.33 Defects in ATG5, ATG7, Atg16l1, Irgm1, and LRRK2 in IECs are also associated with the autophagy pathway,34 manifesting as abnormal Paneth cell morphology and function, affecting the antimicrobial response of Paneth cells and potentially influencing intestinal bacterial infections.
①②: Endoplasmic reticulum (ER) stress enhances autophagy; excessive and unresolved ER stress in intestinal epithelial cells (IECs) promotes intestinal inflammation, while autophagy can alleviate ER stress in various inflammatory and immune diseases. ③⑧: Autophagy inhibits inflammasome activation, reduces caspase-1 (CASP1) activity, and decreases the production of IL-1β and IL-18. ④: Activation of inflammasomes inhibits autophagy and impairs the elimination of autophagy-mediated pro-inflammatory mediators, exacerbating inflammation. ⑤⑥: Autophagy limits ROS accumulation, which in turn inhibits autophagy. The loss of autophagy affects ROS levels, resulting in increased IL-8 secretion, intestinal stem cell (ISCs) recovery defects, and heightened susceptibility to intestinal infections. ⑦: ROS induces the activation of inflammasomes and aggravates intestinal inflammation. ERS, endoplasmic reticulum stress; NLRP3, NOD-like receptor protein 3; ROS, Reactive oxygen species. (This figure was created by Figdraw).
Intestinal epithelial autophagy and ROS-induced oxidative stress
The intestinal mucosa, and specifically intestinal stem cells (ISCs), require autophagy to mitigate ROS-induced oxidative stress.35,36 ROS are unavoidable by-products of cellular metabolism, mainly originating from redox reactions in organelles such as mitochondria, the endoplasmic reticulum, and cell membranes. In the inflammatory state, the activity of the NOX family is enhanced, leading to overproduction of ROS, which plays an important role in the pathogenesis of IBD. NOX4-dependent ROS production correlates with autophagy suppression, aggravating IBD inflammation.37 Studies have shown that NOX4 exacerbates IBD by mediating inflammatory macrophages and promoting damage to the intestinal mucosal barrier.38
ROS play a dual role in autophagy regulation. Moderate levels of ROS can activate autophagy as a protective mechanism to remove damaged organelles and proteins, whereas excessive ROS can impair autophagic flux, leading to cellular dysfunction.39 In IBD, ROS overproduction is closely associated with intestinal epithelial cell damage, immune activation, and gut microbiota dysbiosis.40 ROS can modulate autophagy through multiple pathways, including inhibition of mTOR signaling and activation of AMPK and Beclin-1. Furthermore, ROS-induced autophagy can interact with NLRP3 inflammasome activation, forming a complex network that influences IBD pathogenesis. Autophagy protects intestinal epithelial cells from oxidative damage by decreasing intracellular ROS levels through the removal of excess ROS-producing mitochondria.4,5,41,42 The overproduction of ROS is closely related to damage to intestinal epithelial cells, immune activation, and dysregulation of the gut microbiota.43,44 Studies have shown that patients with IBD experience significant oxidative stress in the gut, leading to increased cellular damage and inflammatory responses.45
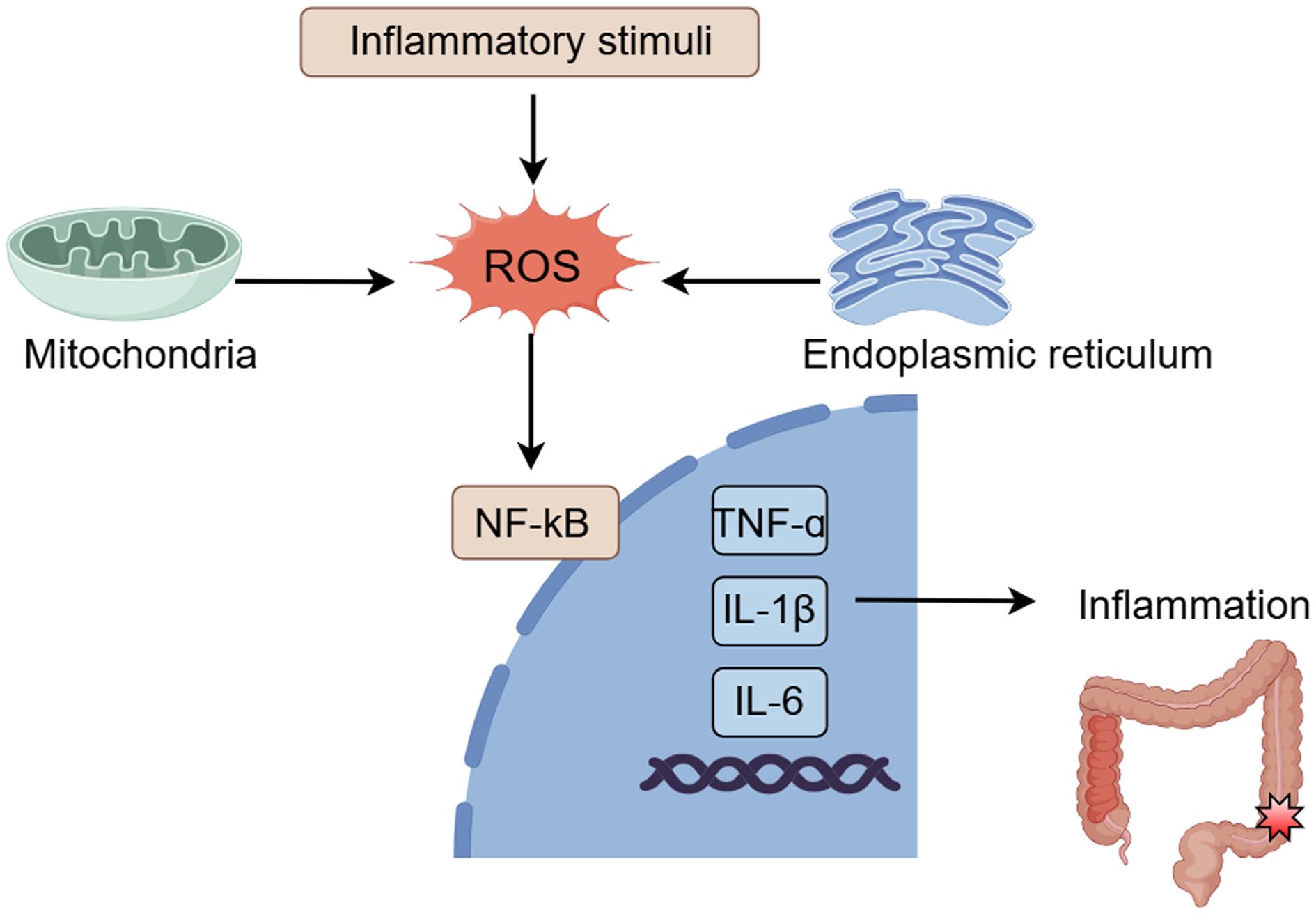
Additionally, ROS can further exacerbate intestinal inflammation by activating multiple signaling pathways, such as NF-κB and mitogen-activated protein kinase pathways, to promote the release of inflammatory cytokines (Fig. 3).46 Autophagy and ROS are also involved in the maintenance of ISCs.35 In fact, Atg5ΔIEC mice exhibited fewer ISCs after irradiation compared to wild type (WT) mice, associated with higher ROS levels and defects in intestinal recovery associated with ISCs. Treatment of Atg5ΔIEC mice with an antioxidant repaired the intestinal recovery deficiency, suggesting that autophagy maintains ISCs and intestinal regeneration by reducing excess ROS levels.35 Thus, autophagy deficiency increases susceptibility to intestinal infections.
The expression of pro-inflammatory genes leads to the production of inflammatory cytokines and other signaling molecules, contributing to intestinal mucosal inflammation. NF-κB, nuclear factor-κB; ROS, Reactive oxygen species. (This figure was created by Figdraw).
In summary, controlling ROS production may be a key strategy for treating IBD. Researchers are exploring the use of antioxidants to reduce inflammation by decreasing oxidative stress in the gut.47,48 Recently, orally administered Inflamed Colon-Targeted Nanotherapeutics (ICANs) have been developed. These nanotherapeutics consist of ROS-scavenging cerium dioxide nanoparticles coated with negatively charged polyacrylic acid. ICANs work by delivering their payload to inflammatory intestinal tissues through interaction with a positively charged protein expression membrane. This targeted approach effectively clears ROS, improves intestinal mucosal inflammation, and enhances autophagy in a DSS-induced colitis model.49 In other studies, oral administration of fullerene nanoantioxidants effectively reduced excess NOX4-dependent ROS in the gut of zebrafish and mouse models, maintaining redox homeostasis and intestinal barrier integrity while improving intestinal and systemic inflammation.37 Additionally, ROS inhibitors such as CoQ10 derivatives and glutathione protect the gut from bacterial infections by regulating ROS levels, promoting mucosal repair, and protecting the gut from bacterial infections.50,51 Therefore, ROS is not only an important signaling molecule involved in the pathophysiological changes of IBD but also a potential therapeutic target. Regulating ROS levels to restore autophagy function may provide new ideas and directions for IBD treatment.
Intestinal epithelial autophagy and endoplasmic reticulum stress
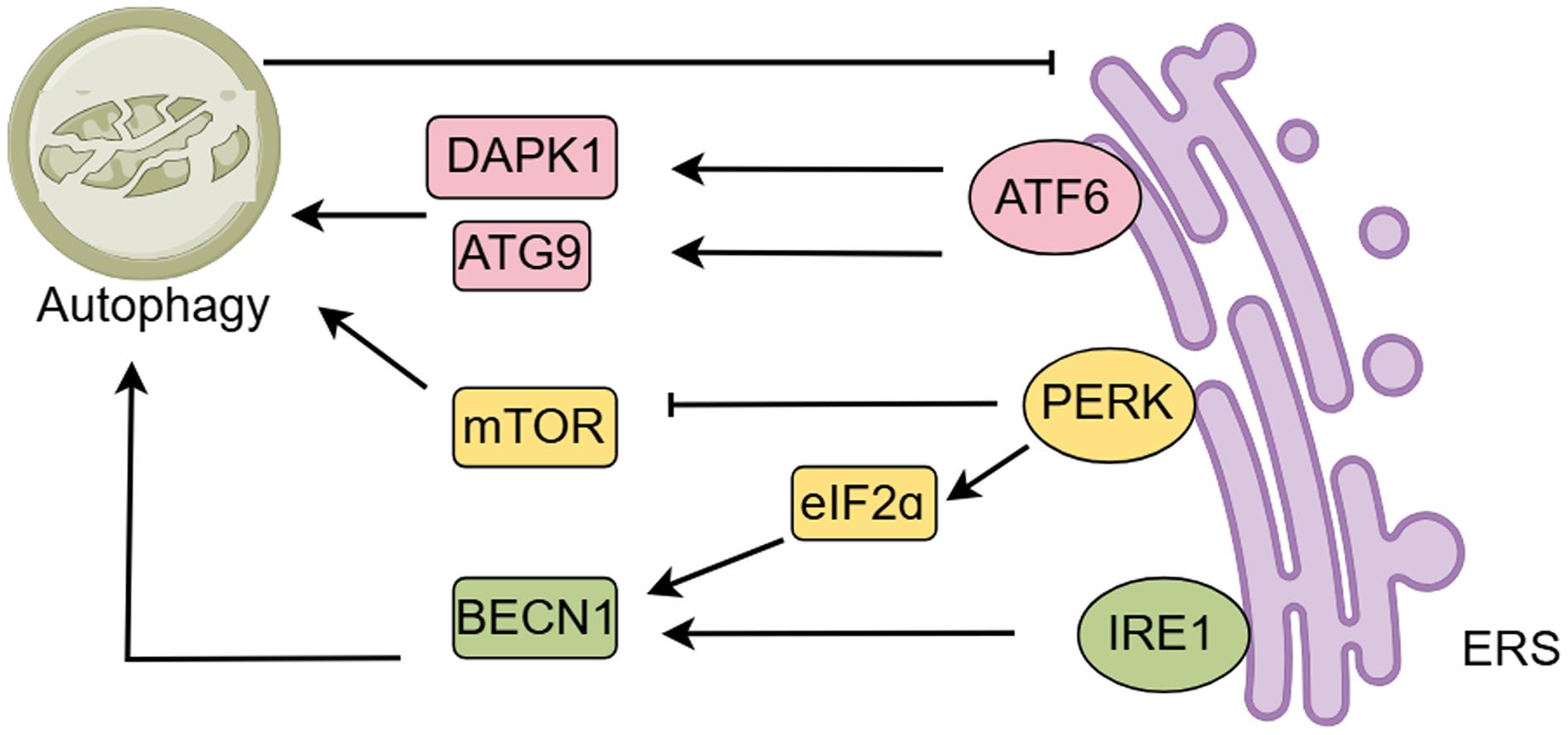
Autophagy, the unfolded protein response, and ERS are interrelated at multiple levels (Fig. 4).52 Endoplasmic reticulum stress can activate autophagy to help cells cope with the accumulation of unfolded proteins.53,54 For example, the protein kinase R-like endoplasmic reticulum kinase (PERK) pathway is activated under ERS conditions, promoting the expression of autophagy-related genes, which in turn enhances autophagic activity. Additionally, ERS affects the autophagy process through signaling pathways such as inositol-requiring enzyme-1 (IRE1) and activating transcription factor 6. Activation of these pathways not only helps to remove damaged organelles and proteins but also maintains cellular homeostasis and prevents apoptosis.55,56 However, excessive ERS can inhibit autophagy, leading to the accumulation of intracellular damaging substances that further exacerbate intestinal inflammation.6,57 Therefore, regulating the balance between ERS and autophagy may offer a novel strategy for treating IBD. For example, certain natural products, such as inulin, have been shown to reduce ERS, promote autophagic activity, and improve IBD symptoms.58
PERK activates ATF4 by phosphorylating eukaryotic initiation factor 2α (eIF2α) via auto-phosphorylation, inhibiting mTOR phosphorylation to activate autophagy. IRE1 and PERK promote autophagy by upregulating Beclin-1. Autophagy and ERS mutually regulate each other. ERS can promote autophagy, and when ERS is overactive, activated autophagy can inhibit ERS, thereby reducing apoptosis in intestinal epithelial cells (IECs), downregulating pro-inflammatory cytokines, and protecting the intestinal mucosal barrier. ATG9, Autophagy-related protein 9; BECN1, Beclin-1; ERS, endoplasmic reticulum stress; mTOR, Mechanistic Target of Rapamycin. (This figure was created by Figdraw).
Excessive and unresolved ERS in intestinal epithelial cells contributes to intestinal inflammation.59 Activation of receptors such as TLR4 and TLR2 can enhance ERS through the p38 mitogen-activated protein kinase pathway, exacerbating the inflammatory response in the gut.6,60 ERS may also increase intestinal permeability by affecting the intestinal barrier, further promoting dysbiosis of the gut microbiota and creating a vicious cycle.7,61 Modulating ERS-related signaling pathways such as IRE1 and PERK is also considered a potential therapeutic strategy. Although these pathways activate autophagy,62,63 their sustained activation under pathological ERS conditions may paradoxically exacerbate intestinal dysfunction and inflammation.63 Targeted inhibition in a context-dependent manner could attenuate maladaptive signaling cascades while preserving basal autophagy, thereby restoring epithelial homeostasis and reducing inflammation.64 Autophagy reduces the incidence of ERS by removing unfolded proteins from the endoplasmic reticulum.65,66 Autophagy can also alleviate ERS and promote cell survival by regulating ERS-related signaling pathways.67,68 In IBD, autophagy preserves intestinal mucosal barrier integrity by mitigating ERS and regulating goblet cell mucin production.69 Experimental studies have demonstrated that sustained Beclin 1-mediated autophagic activation in murine models enhances mucus barrier integrity through ERS-dependent modulation of goblet cell mucin biosynthesis, resulting in a hyperconcentrated mucus layer with reduced macromolecular permeability.70 Recent studies have shown that Portulaca oleracea L regulates ERS and autophagy through the PERK-eukaryotic initiation factor 2α/Beclin1-microtubule-associated protein light chain 3II pathway, promoting colitis healing and improving intestinal inflammation.15 In summary, mechanistic studies on the ERS-autophagy regulatory network will elucidate novel therapeutic approaches for IBD.
Intestinal epithelial autophagy with NLRP3 inflammasome
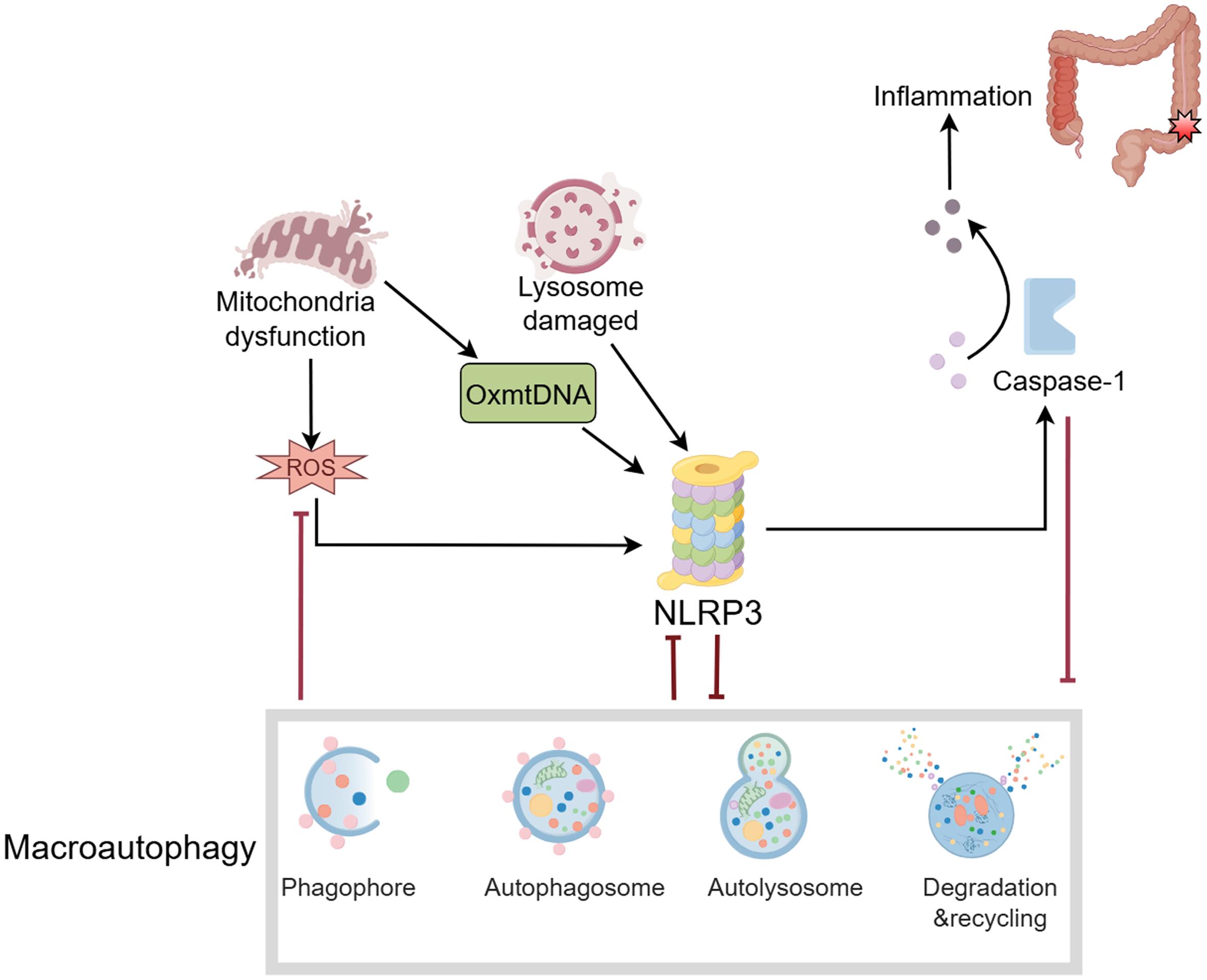
The NLRP3 inflammasome is involved in regulating multiple inflammatory responses and consists of the NLRP3 protein, ASC, and caspase-1. It senses a variety of endogenous and exogenous stimuli to activate downstream inflammatory responses (Fig. 5).71 NLRP3 inflammasome activation promotes IL-1β/IL-18 maturation and is associated with IBD pathology through intestinal barrier dysfunction, immune cell recruitment, and overproduction of inflammatory cytokines.72 In DSS-induced mouse models, inhibiting the NLRP3 inflammasome significantly reduces disease symptoms, such as weight loss and colon shortening, while also lowering pro-inflammatory cytokine levels.73 It has also been suggested that NLRP3 activation may be indirectly influenced by modulating the intestinal microbiota, improving the pathological state of IBD.74
NLRP3 inflammasome activation triggers caspase-1 activation, which cleaves IL-1β and IL-18 to produce their active forms. Active IL-1β and IL-18 contribute to intestinal inflammation. NLRP3, NOD-like receptor protein 3; ROS, reactive oxygen species. (This figure was created by Figdraw).
In macrophages, autophagy plays a crucial role in regulating NLRP3 inflammasome activation. Dysfunctional autophagy in macrophages leads to aberrant NLRP3 activation, resulting in excessive release of IL-1β and IL-18, which worsens intestinal inflammation.75,76 Furthermore, autophagy in dendritic cells can modulate NLRP3 inflammasome activity by degrading damaged mitochondria, which are a major source of ROS, thereby reducing inflammation.77 As a hallmark protein of autophagy, p62/SQST binds to NLRP3 and promotes its autophagic degradation, inhibiting its activation.78 The CD susceptibility genes PTPN2 and PTPN22 are also involved in regulating autophagy and inflammasome activity.79,80 PTPN2 regulates inflammasome activation and IL-1β secretion by limiting the phosphorylation of the adaptor molecule ASC,79 while PTPN22 decreases IL-1β secretion by limiting NLRP3 degradation. Myeloid-specific Ptpn2 deficiency exacerbates DSS-induced colitis by amplifying inflammasome activation and increasing IL-1β production.80
The NLRP3 inflammasome plays a complex role in IBD, exhibiting both protective and pro-inflammatory effects depending on the environment. In certain models, such as DSS-induced and TNBS-induced colitis, NLRP3 deficiency attenuates colitis by reducing pro-inflammatory cytokine production, including IL-1β and IL-18. However, other studies show that NLRP3 inflammasome activation can have protective effects, particularly in maintaining epithelial integrity and regulating intestinal immune responses. Insufficient NLRP3 activity may impair bacterial clearance and disrupt immune regulation, potentially exacerbating inflammation, while excessive NLRP3 activation leads to overproduction of pro-inflammatory cytokines, causing tissue damage and chronic inflammation.4,81 In the context of autophagy, NLRP3 inflammasomes are involved in regulating autophagy activation. Under inflammatory conditions, NLRP3 inflammasome directly interacts with mTOR to induce its kinase activation loop phosphorylation (Thr2446/Ser2448),82 suppressing unc-51 like autophagy activating kinase 1-dependent autophagosome formation and disrupting the selective autophagic degradation of damaged mitochondria, thereby sustaining inflammatory pathology through unresolved cytokine storms.83 In DSS-induced mouse models of colitis or IL-10−/− mice, hypoxia reduces intestinal inflammation by reducing NLRP3-mTOR binding, activating autophagy-mediated degradation of NF-κB signaling mediators, and decreasing pro-inflammatory gene expression. These studies show the reciprocal regulation between autophagy and inflammasomes and suggest that autophagy-inducing NLRP3 inhibitors or inflammasome-inhibiting autophagy activators could serve as potential therapeutic agents for IBD treatment.84
Therapeutic measures based on the mechanism of autophagy
Targeted autophagy as a treatment is an active area of research in various fields of medicine, including IBD, cancer, neurodegenerative diseases, and infectious diseases. Preclinical studies have shown that many current treatments for IBD involve mechanisms that regulate autophagy (Table 1).15,49-51,58,85-95 Autophagy-based therapies for IBD are prioritized based on their mechanistic coverage, targeting interconnected pathways such as ROS/NLRP3 inflammasome activation, ERS, and mitochondrial dysfunction.
Autophagy-inducing therapy for inflammatory bowel diseases (IBD) drugs
| Type | Drug candidate | Mode of action | References |
|---|---|---|---|
| NOD-like receptor protein 3 (NLRP3) Inflammasome inhibitors | Small molecule AMP-activated protein kinase (AMPK) activator | Prevent colitis by inducing autophagy to degrade the NLRP3 inflammasome complex in macrophages | 87 |
| Ginsenoside Rd | Alleviate Dextran Sulfate Sodium (DSS)-induced colitis by inducing p62-mediated mitophagy to induce NLRP3 inflammasome inactivation | 85 | |
| Palmatine | Ameliorates DSS-induced colitis by promoting mitochondria-mediated NLRP3 inflammasome inhibition | 86 | |
| Metformin in combination with MCC950 | Sabre and El-Kader demonstrated that autophagy inhibits the NLRP3 inflammasome and modulates interactions to alleviate DSS-induced colitis | 89,90 | |
| Nicotinic acetylcholine receptor agonists | DSS-induced colitis can be alleviated by triggering the cholinergic anti-inflammatory pathway, which in turn affects NLRP3 inflammasome-associated interleukin (IL)-1β and IL-18 production by inhibiting the signaling pathway | 91 | |
| Cannabinoid receptor2 agonist | Induction of mediated autophagy, thereby inhibiting the NLRP3 inflammasome, ameliorates DSS-induced colitis | 88 | |
| mammalian target of rapamycin (mToR) inhibitors | Sirolimus minor | Promotes intestinal mucus recovery | 92 |
| Compound everolimus | Reduces lymphocytic infiltration | 93 | |
| Rapamycin | Regulates intestinal flora and alleviates DSS-induced colitis | 94 | |
| Glutamine | Inhibits apoptosis and inhibits mTOR pathway | 95 | |
| ROS inhibitors | MitoQ | Targeted removal of ROS in mitochondria promotes mucosal repair | 50 |
| Glutathione | By regulating ROS levels and mitochondrial function, it affects the production of IL-22 and protects the intestine from bacterial infection | 51 | |
| Orally Administrated Inflamed Colon-Targeted Nanotherapeutics (ICANs) | Eliminate ROS by targeted delivery to inflammatory intestinal tissue with a positively charged protein expression membrane to ameliorate DSS-induced colitis | 49 | |
| Endoplasmic reticulum stress regulators | Inulin | Restore intestinal flora, regulate endoplasmic reticulum stress, and improve mucosal inflammation | 58 |
| Portulaca oleracea L(POL) | Regulates endoplasmic reticulum stress and autophagy through the (PERK)-2α(eIF2α)/Beclin1- (LC3II) pathway, thereby promoting colitis healing | 15 |
Several autophagy modulators have been reported to attenuate IBD by inhibiting the activation of the NLRP3 inflammasome. For example, Liu et al. found that Ginsenoside Rd, a tetracyclic triterpenoid derivative, alleviates DSS-induced colitis in a UC model by inducing p62-mediated mitophagy to inactivate the NLRP3 inflammasome.85 Another natural derivative, Palmatine, ameliorates DSS-induced colitis by promoting mitochondria-mediated NLRP3 inflammasome inhibition.86 The small-molecule AMPK activator andrographolide, an herbal extract isolated from Southeast Asian plants, degrades the NLRP3 inflammasome complex in macrophages by inducing autophagy, ameliorating DSS-induced colitis in mice.87 Cannabinoid receptor 2, a G protein-coupled receptor predominantly located on immune cells, has been used in the treatment of IBD, and agonist-mediated activation of cannabinoid receptor 2 increases autophagy, thereby inhibiting the activation of the NLRP3 inflammasome and attenuating DSS-induced colitis in mice.88 Conversely, inhibition of NLRP3 inflammasomes may be an effective strategy to promote autophagy activation. There are also ROS inhibitors and ERS regulators mentioned, such as targeted nanotherapeutics (ICANs), glutathione, inulin, and Portulaca oleracea L.
In conclusion, these strategies address multiple pathological axes, enhancing therapeutic efficacy by disrupting cross-amplifying inflammatory loops. Future research should focus on optimizing multi-mechanistic agents and validating their clinical translation to advance precision IBD treatment.
Perspectives
Future studies should integrate multi-omics approaches to tailor therapies to individual patient profiles. While antioxidants and NLRP3 inhibitors show promise, their long-term safety and efficacy in humans need rigorous validation. Novel drug delivery systems (e.g., ROS-targeted nanotherapeutics) and combination therapies (e.g., autophagy inducers with biologics) warrant exploration. Most evidence derives from animal models; human studies are limited. Clinical trials should assess autophagy-modulating agents in diverse IBD subtypes to identify context-dependent benefits. We hypothesize that future research could prioritize elucidating autophagy-immune cell crosstalk, refining targeted drug delivery systems, and validating clinical applications across IBD subtypes.
Conclusions
Autophagy serves as a critical regulator in the pathogenesis of IBD by maintaining intestinal homeostasis through dynamic interactions with ROS, NLRP3 inflammasome, and ERS. The ROS/NLRP3 axis acts as a central driver of intestinal inflammation, where excessive ROS generation activates the NLRP3 inflammasome, triggering the secretion of pro-inflammatory cytokines, including IL-1β and IL-18, and amplifying mucosal injury. Autophagy mitigates these pathological effects through ROS scavenging, selective degradation of NLRP3 components, and attenuation of mitochondrial dysfunction. Furthermore, autophagy ameliorates ERS by facilitating the clearance of misfolded proteins and modulating key unfolded protein response signaling pathways, such as PERK, IRE1, and activating transcription factor 6, thereby reinforcing intestinal barrier integrity. Therapeutic strategies targeting autophagy induction (e.g., AMPK activators, mTOR inhibitors), ROS/NLRP3 axis inhibition (e.g., antioxidants, NLRP3 inhibitors), and ERS regulation (e.g., inulin, Portulaca oleracea L.) demonstrate promising efficacy in preclinical IBD models. These findings underscore autophagy as a critical adaptive mechanism and a potential therapeutic target for IBD. Genetic and environmental factors, such as microbiota composition and host genetics (e.g., ATG16L1, NOD2 variants), likely influence autophagy-IBD interactions.
Collectively, autophagy regulates IBD pathogenesis by balancing ROS/NLRP3 inflammasome activation and endoplasmic reticulum stress, thereby preserving intestinal barrier integrity. Targeting autophagy induction, ROS/NLRP3 inhibition, and ERS modulation shows therapeutic potential.
Declarations
Acknowledgement
None.
Funding
This article was supported by the following programs to Prof. Shi-xue Dai: the High-Level Personnel Program of Guangdong Provincial People’s Hospital (2021DFJH0008/KY012021458), Starting Program for National Natural Science Foundation of China at Guangdong Provincial People’s Hospital (8207034250), National Natural Science Foundation of China (NSFC, No. 81300370), Natural Science Foundation of Guangdong (NSFG, No. 2018A030313161), General Program (No. 2017M622650), Special Support Program (No. 2018T110855) from the China Postdoctoral Science Foundation (CPSF).
Conflict of interest
Dr. Shixue Dai serves as an editorial board member of Nature Cell and Science. The authors have no other conflicts of interest related to this publication.
Authors’ contributions
Contributed to study concept and design (BP, XW, SD), acquisition of the data (BP, XW, SD, TT), assay performance and data analysis (BP, XW, SD, TT), drafting of the manuscript (BP, XW, TT, LW, YX SD), and critical revision of the manuscript (BP, XW, TT, LW, YX, SD). All authors made significant contributions to this study and approved the final manuscript.

 Author information
Author information