Introduction
Myopia, commonly known as nearsightedness, is a prevalent eye condition affecting approximately one-third of the global population.1,2 In recent years, its prevalence has been increasing rapidly, particularly among children and adolescents.3 The high incidence of myopia in younger populations has raised concerns about the potential long-term complications of high myopia, such as retinal detachment, glaucoma, and cataracts. Consequently, there is growing research interest in strategies to prevent myopia progression.4
Several interventions have shown promise in slowing myopia progression, including increased outdoor activities, greater exposure to natural light, and reduced time spent on near-work activities.5 Additionally, low-dose atropine eye drops and orthokeratology have demonstrated efficacy in mitigating myopia progression.6,7
Low-dose atropine eye drops (0.01%) have been proposed as an effective strategy for controlling myopia progression. Studies have shown that these drops can reduce myopia progression in children and adolescents by up to 50%.8 Orthokeratology, also known as Ortho-K, is a non-invasive method that employs specially designed gas-permeable contact lenses to temporarily reshape the cornea, allowing for clear vision without the need for glasses or contact lenses during the day. Clinical trials have reported that Ortho-K lenses can slow myopia progression in children and young adults by up to 59%.9
Despite these promising results, the comparative effectiveness of low-dose atropine eye drops, orthokeratology, and their combined use in controlling myopia progression remains a topic of debate.10–12 To address this, we conducted a retrospective cohort study to compare the effectiveness of 0.01% atropine eye drops, orthokeratology, and their combination in controlling myopia progression. This research aimed to provide valuable insights into the comparative effectiveness of these interventions, guiding clinicians in selecting the most appropriate management strategies for patients with myopia.
Material and methods
Ethics statement
The study protocol was approved by the Institutional Review Board and Ethics Committee of Visionly Plus Eye Hospital (Beijing, China, No. 202102) and Parkway Gleneagles Medical and Surgical Center (Shanghai, China, No. 202101). The study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all participants’ legal guardians.
Study design
This retrospective cohort study was conducted across multiple ophthalmic centers. Shenton Health Hong Qiao Medical Center (SH), Parkway Gleneagles Medical and Surgical Center (PG), and Visionly Plus Eye Hospital (VP) participated in the study. VP, located in Beijing, exclusively served Chinese patients, while PG and SH, both located in Shanghai, served both Chinese and Caucasian patients.
Participants
Children who visited SH, PG, or VP between January 2021 and December 2022 and met the following criteria were included in this retrospective study: (A) Aged between eight and 18 years; (B) cycloplegic spherical equivalent between −1.00 and −6.00 diopters; (C) astigmatism of ≤1.50 D in both eyes; (D) no other serious eye diseases, such as cataract, retinopathy of prematurity, glaucoma, or connective tissue disorders associated with myopia, such as Stickler or Marfan syndromes; (E) follow-up duration of at least 12 months; and (F) parental informed consent obtained. Exclusion criteria: (A) Parents and pediatric patients who did not consent to cycloplegic refraction; (B) individuals with myopia exceeding 6.25 diopters; and (C) patients with myopia accompanied by hereditary eye diseases or retinopathy of prematurity.
Treatments
Four treatment options were offered to the children and their parents: (1) No treatment (control group–glasses only), (2) orthokeratology, (3) 0.01% atropine eye drops, and (4) combined orthokeratology with 0.01% atropine. The advantages and disadvantages of all four options were explained to the patients and their guardians, who then self-selected their treatment. Alpha overnight orthokeratology lenses (Alpha Corporation, Japan) were used in this study, and 0.01% atropine eye drops were provided by the Eye and ENT Hospital of Fudan University (Shanghai, China).
Examinations
Comprehensive ophthalmic examinations were performed at every visit. The technique used was consistent among ophthalmologists across all three centers. The anterior and posterior segments of the eyes were assessed using a slit lamp and slit lamp lenses (Digital Wide Field, Volk, USA).
Full cycloplegia was initially achieved by administering three drops of cycloplegic eye drops (Mydrin P, Tropicamide 0.5%, phenylephrine HCl 0.5%; Santen Pharmaceutical, Shiga, Japan) at 5-m intervals, followed by a 30-m wait. Refractive error (sphere and cylinder) was measured with a desktop autorefractor (KR-8800; Topcon Corporation, Japan) at baseline and repeated after six and 12 months. Measurements were conducted for both eyes by experienced ophthalmologists or optometrists. The spherical equivalent (SE) was calculated as the sphere plus half the cylinder. For the control group and patients using atropine alone, full cycloplegia was performed at each visit to ensure accurate measurement of myopia.
Axial length (AL) was measured using an optical biometry device (AL-Scan Optical Biometer, Nidek, Japan). At least five successive measurements were taken during each visit, with the mean used as the final AL value. These measurements were performed by well-trained ophthalmologists or optometrists.
Statistical analysis
The rate of AL progression was calculated as the difference between the AL at the end of the follow-up and the AL at the first follow-up, divided by the interval between these examinations. Similarly, the rate of myopia progression was calculated as the difference between the SE at the end of the follow-up and the SE at the first follow-up, divided by the time between the first and last measurements. Data were analyzed using the R programming language (version 4.1.3). ANOVA was used to compare the rates of AL and SE progression between groups. Statistical significance was set at p < 0.05.
Results
Study sample characteristics
Table 1 outlines the basic characteristics of our study, which included 736 eyes of 736 myopic children: 265 in the control group, 155 in the orthokeratology group, 181 in the atropine group, and 135 in the combined group. Only the right eyes were analyzed, as both eyes were predominantly isotropic (Pearson correlation coefficient ranged from 0.95 to 0.98 between the right and left eyes across all four groups). Of the participants, 584 (79.35%) were Chinese, and 152 (20.65%) were Caucasian. Additionally, 361 (49.05%) were males, and 375 (50.95%) were females. Baseline characteristics, including age, gender, race, spherical equivalent, and axial length, did not significantly differ among the four groups (control, 0.01% atropine eye drops, orthokeratology, and combined 0.01% atropine eye drops with orthokeratology).
Demographic and biometric data at baseline for the four groups
| Control | Atropine | Ortho-K | Atropine + Ortho-K | p-value | |
|---|---|---|---|---|---|
| N | 265 | 181 | 155 | 135 | |
| Baseline age | 8.66 ± 2.46 | 8.71 ± 2.23 | 9.08 ± 2.25 | 9.67 ± 2.65 | 0.276 |
| Gender | |||||
| Male | 122 (46.04%) | 96 (53.04%) | 73 (47.10%) | 70 (51.85%) | 0.833 |
| Female | 143 (53.96%) | 85 (46.96%) | 82 (52.90%) | 65 (48.15%) | 0.693 |
| Race | |||||
| Chinese | 209 (78.87%) | 144 (79.56%) | 124 (80.00%) | 107 (79.26%) | 0.522 |
| Caucasian | 56 (21.13%) | 37 (20.44%) | 31 (20.00%) | 28 (20.74%) | 0.628 |
| Spherical equivalent (D) | 2.61 ± 0.59 | 2.82 ± 0.63 | 3.12 ± 0.56 | 3.07 ± 0.72 | 0.177 |
| PCC between the right eye and left eye | 0.98 (p < 0.001) | 0.98 (p < 0.001) | 0.96 (p < 0.001) | 0.98 (p < 0.001) | |
| Axial length (mm) | 24.77 ± 0.62 | 24.92 ± 0.57 | 25.28 ± 0.65 | 25.19 ± 0.56 | 0.229 |
| PCC between the right eye and left eye | 0.96 (p < 0.001) | 0.95 (p < 0.001) | 0.98 (p < 0.001) | 0.95 (p < 0.001) |
Rate of axial length elongation
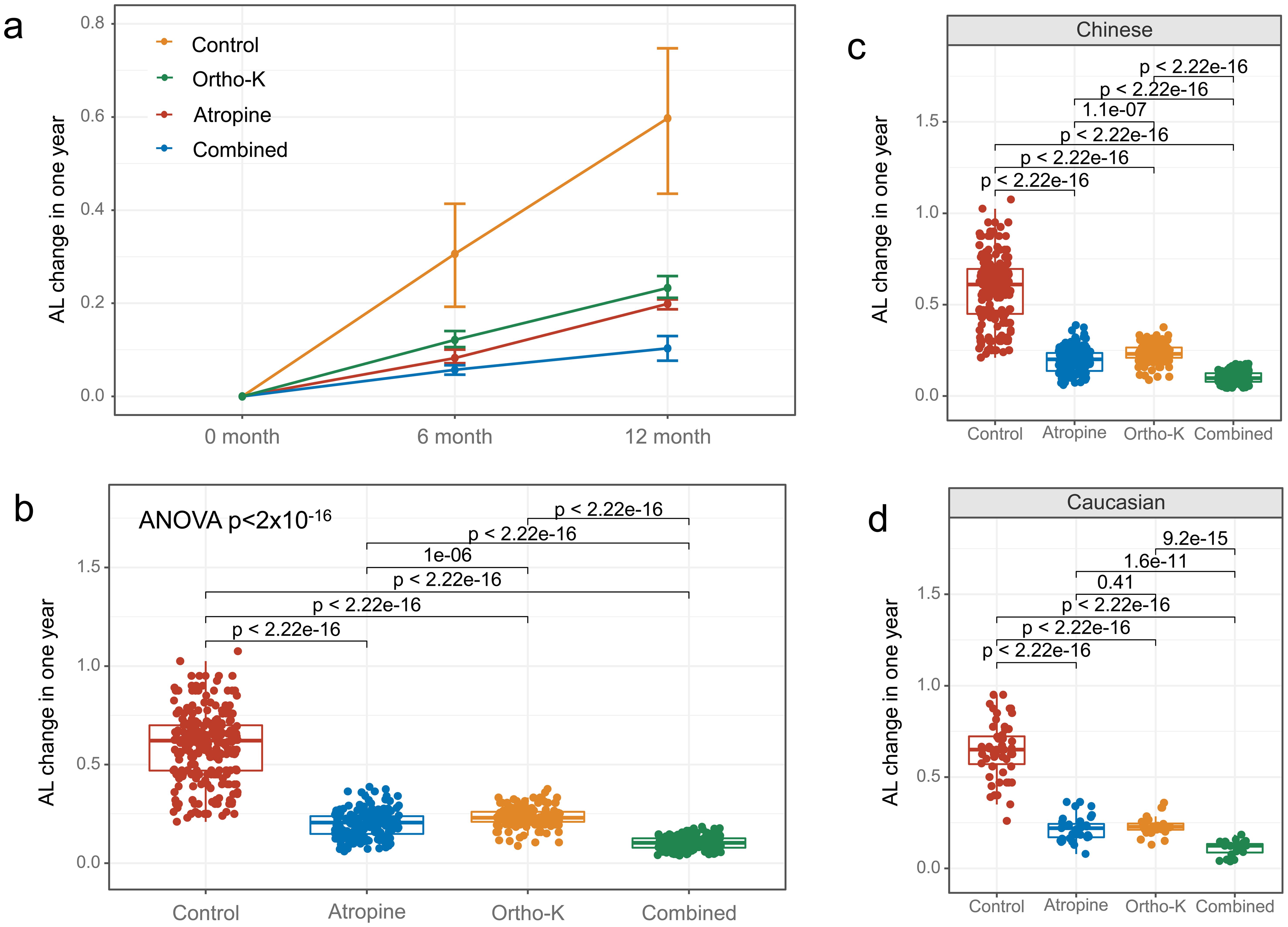
Significant differences were observed in the rate of AL elongation among the treatment groups (ANOVA analysis: SS = 26.10, F = 696.71, p = 1.20 × 10−191). The one-year rates of AL elongation were significantly lower in the 0.01% atropine eye drops group (0.20 ± 0.07 mm, p < 0.001), the orthokeratology group (0.23 ± 0.05 mm, p < 0.001), and the combined 0.01% atropine eye drops with orthokeratology group (0.10 ± 0.04 mm, p < 0.001) compared to the control group (0.60 ± 0.17 mm, Fig. 1a, b). Similar trends were observed in both Chinese and Caucasian children (Fig. 1c, d). The combined 0.01% atropine eye drops with the orthokeratology group exhibited the most effective control of AL elongation. Moreover, 0.01% atropine eye drops alone were more effective than orthokeratology alone (p < 0.001).
The rate of axial length elongation over one year was measured in different treatment groups and the control group. (a, b) The rates of axial length elongation were significantly lower in the 0.01% atropine eye drops group, orthokeratology group, and combined 0.01% atropine eye drops with orthokeratology group compared to the control group. Similar results were observed in both Chinese (c) and Caucasian (d) children. The combined 0.01% atropine eye drops with the orthokeratology group showed the best control of axial length elongation, while the 0.01% atropine eye drops alone were more effective than orthokeratology alone.
Rate of spherical equivalent progression
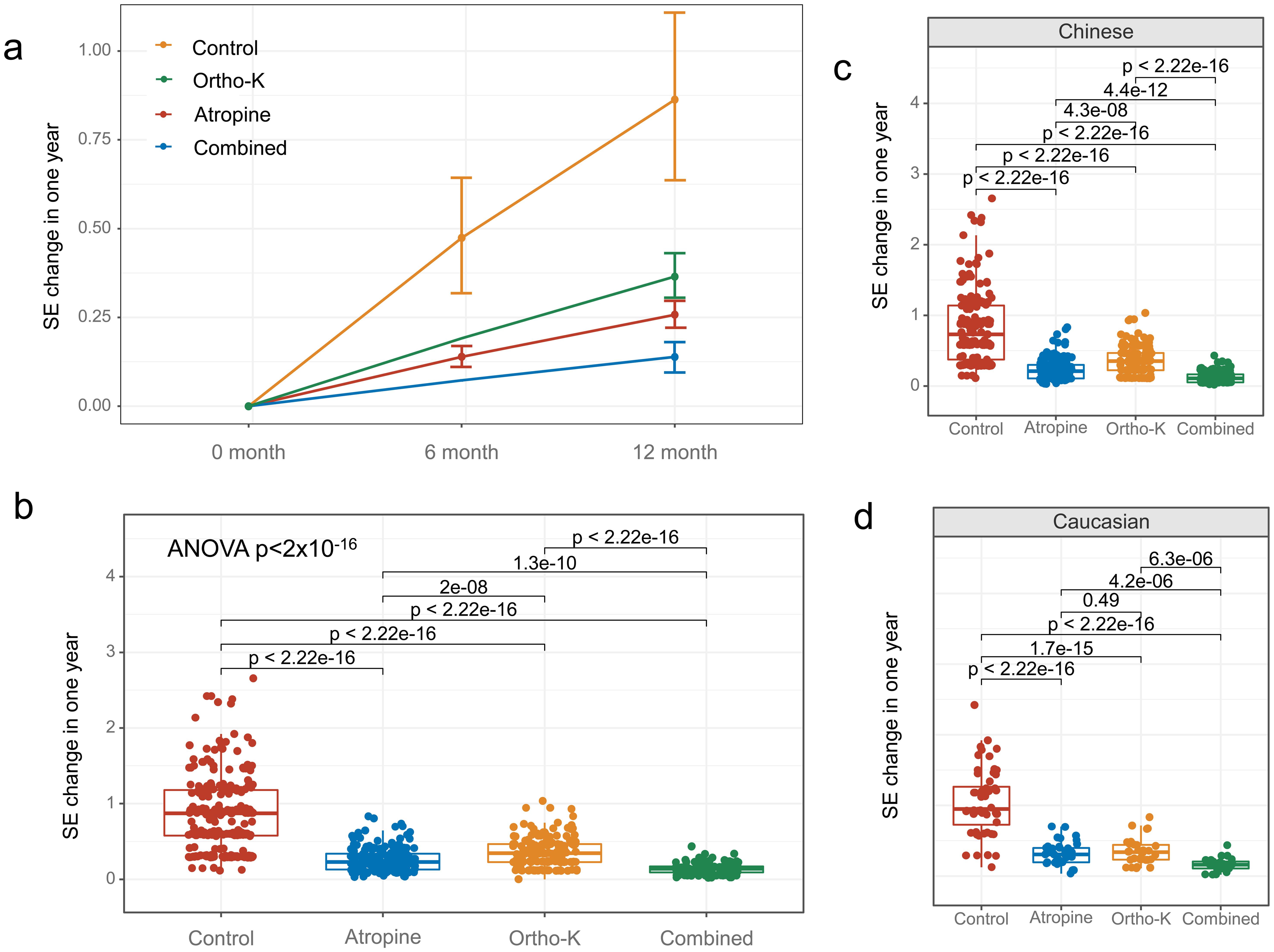
Significant differences were also observed in the rate of SE progression among the treatment groups (ANOVA analysis: SS = 63.54, F = 204.35, p = 2.23 × 10−90). The one-year rates of myopia progression (SE) were significantly slower in the 0.01% atropine eye drops group (0.26 ± 0.12 D, p < 0.001), the orthokeratology group (0.36 ± 0.17 D, p < 0.001), and the combined 0.01% atropine eye drops with orthokeratology group (0.14 ± 0.08 D, p < 0.001) compared to the control group (0.86 ± 0.23 D, Fig. 2a, b). Similar trends were observed in both Chinese and Caucasian children (Fig. 2c, d). The combined 0.01% atropine eye drops with the orthokeratology group showed the best control of SE progression. Furthermore, 0.01% atropine eye drops alone were more effective than orthokeratology alone (p < 0.001).
The graph illustrates the mean myopia progression in diopters over one year across four groups: 0.01% atropine eye drops, orthokeratology, combined 0.01% atropine eye drops with orthokeratology, and the control group. The results show that myopia progression in the three treatment groups was significantly slower compared to the control group (a, b). The combined 0.01% atropine eye drops with the orthokeratology group exhibited the best control of myopia progression. The same trends were observed in both Chinese (c) and Caucasian (d) children. However, the spherical equivalent was not assessed for the orthokeratology and combined groups at six months, as children needed to stop wearing orthokeratology lenses to check the spherical equivalent.
Discussion
Given the high prevalence of myopia and its potential impact on vision, it is crucial to identify effective treatments that can slow the progression of myopia in children.3 The present study investigated the effect of 0.01% atropine eye drops, orthokeratology, and their combination on the rate of axial length elongation and myopia progression in children with myopia. The study included 736 eyes of 736 myopic children, with a balanced distribution of gender, race, and baseline characteristics among the four groups (control, 0.01% atropine eye drops, orthokeratology, and combined 0.01% atropine eye drops and orthokeratology).
Our findings revealed that all three interventions—0.01% atropine eye drops, orthokeratology, and their combination—were effective in slowing the rate of axial length elongation and myopia progression in children. The combined use of 0.01% atropine eye drops and orthokeratology demonstrated the best control of axial length elongation and myopia progression. Comparatively, 0.01% atropine eye drops alone were more effective than orthokeratology alone in controlling both axial length elongation and myopia progression.
The effectiveness of atropine eye drops in slowing myopia progression has been previously reported in several studies.6,13,14 Atropine works by inhibiting the muscarinic receptors in the eye, thereby reducing accommodation and pupil constriction. This, in turn, decreases axial length elongation and the progression of myopia.15 Our findings support the effectiveness of 0.01% atropine eye drops in controlling myopia progression, with a reduction in the rate of axial length elongation to 0.20 ± 0.07 mm in one year. However, the Pediatric Eye Disease Investigator Group study did not support the use of 0.01% atropine eye drops to slow myopia progression or axial elongation in U.S. children, suggesting a racial difference in response to atropine.16
Orthokeratology involves the use of specially designed contact lenses to reshape the cornea temporarily.17 These lenses are worn overnight, allowing clear vision during the day without the need for glasses or contact lenses.18,19 The effectiveness of orthokeratology in controlling myopia progression has been reported in several studies.19,20 Our study also showed a significant reduction in the rate of axial length elongation in the orthokeratology group (0.23 ± 0.05 mm) compared to the control group. Additionally, ongoing studies, such as the China Alliance of Research in High Myopia study, may provide further insights into the effectiveness of orthokeratology lenses in controlling myopia once their outcomes are available.21
The combination of 0.01% atropine eye drops with orthokeratology has been reported in only a few studies, with mixed results.22–24 Our study found that the combined treatment approach resulted in the slowest rate of axial length elongation (0.10 ± 0.04 mm), indicating its potential for effectively controlling myopia progression. A possible explanation for the enhanced efficacy of the combined treatment is that orthokeratology reduces peripheral hyperopic defocus, which stimulates axial elongation and myopia progression, while atropine eye drops inhibit muscarinic receptors, also implicated in myopia progression.25 Recent research indicates that atropine combined with orthokeratology enhances myopia control efficacy compared to monotherapy in children aged eight to twelve.26 Moreover, younger children may derive greater benefits from orthokeratology.26 The combination of these two treatments may have a synergistic effect on controlling myopia progression, as both approaches target different aspects of the underlying mechanism of myopia.
Interestingly, our study also showed that 0.01% atropine eye drops alone were more effective than orthokeratology alone in controlling axial elongation and myopia progression. This finding aligns with previous studies that reported the efficacy of atropine eye drops in controlling myopia progression.27,28 Atropine eye drops are thought to work by inhibiting the accommodative response and reducing the hyperopic defocus that drives axial elongation and myopia progression. However, atropine eye drops are associated with several side effects, including photophobia, blurred vision, and loss of near vision, which may limit their use in children.6
It is noteworthy that the effectiveness of these treatments was consistent in both Chinese and Caucasian children. The findings of our study align with previous studies that reported a similar effect of atropine eye drops and orthokeratology in controlling myopia progression in both Asian and non-Asian populations.22,29,30 This suggests that the underlying mechanism of myopia progression is similar across different ethnicities and that these treatments may effectively control myopia progression in diverse populations.
The present study has some limitations that should be considered when interpreting the results. Firstly, the study was conducted over a one-year period, and a longer-term follow-up is needed to assess the sustainability of the interventions’ effects on axial length elongation and myopia progression. Secondly, the study did not investigate the mechanisms underlying the interventions’ effects on axial length elongation and myopia progression. Future studies should explore these mechanisms. Thirdly, the study did not investigate the potential adverse effects of the interventions. Future studies should assess the safety of these interventions in children. Furthermore, the participants’ age range of eight to eighteen years is quite broad, and responses may differ significantly between younger and older patients. In future studies, we will further investigate the differential responses to various treatments between the eight–twelve and thirteen–eighteen age groups.
Conclusions
In conclusion, the present study provides evidence for the effectiveness of 0.01% atropine eye drops, orthokeratology, and their combination in controlling axial length elongation and myopia progression in children. The combined use of 0.01% atropine eye drops and orthokeratology demonstrated the most effective control of both axial length elongation and myopia progression. These findings have important implications for the management of myopia in children and suggest that a combination of interventions may be more effective than a single intervention.
Declarations
Acknowledgement
We express our gratitude to Dr. Andrea Sonntag from the Department of Ophthalmology at Parkway Gleneagles Medical and Surgical Center, Shanghai, China, for her valuable suggestions and assistance with this study.
Ethical statement
The study protocol was approved by the Institutional Review Board and Ethics Committee of Visionly Plus Eye Hospital (Beijing, China, No. 202102) and Parkway Gleneagles Medical and Surgical Center (Shanghai, China, No. 202101). The study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all participants' legal guardians.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81200669).
Conflict of interest
One of the authors, Peng Zhou, has served as an executive associate editor for Nature Cell and Science journal since January 2024. The authors declare no other conflicts of interest.
Authors’ contributions
Analysis, writing (PZ), and data collection (DW). All authors agreed on the final edition of this manuscript.

 Author information
Author information