Introduction
Herbal remedies, rich in diverse phytoconstituents, have been invaluable since ancient times for preventing and treating diseases, including cancer, and remain integral to global healthcare.1 Medicinal plants, known for their safety and efficacy, offer a natural alternative to synthetic drugs, with minimal side effects and promising medical outcomes. Catharanthus roseus (L.) Don, commonly called “Nayantara” or “Sadabahar,” is a large evergreen herb from the Apocynaceae family. Its name, derived from Greek, signifies “unadulterated blossom,” with “roseus” reflecting its distinctive rose-colored appearance.2Catharanthus roseus (C. roseus), an ornamental plant with pink (Rosea) and white (Alba) floral varieties, produces vinca alkaloids—nitrogenous compounds crucial for therapeutic uses, particularly derived from pink flowers.3 Canadian scientists Robert Noble and Charles Beer discovered vinca alkaloids in the 1950s. The Vinca genus, which includes V. major L., V. minor L., and V. herbacea Waldst. & Kit., is widely distributed across tropical regions globally, including Southern Europe, North and South America, Asia, Africa, and Australia.4 Vinca alkaloids such as vinblastine and vincristine (C46H58O9N4·H2SO4 and C46H56O10N4·H2SO4) are among the 130 bioactive indole alkaloids in C. roseus. These alkaloids are highly valued for their potent therapeutic effects despite their inherent toxicity.5 Vinca alkaloids, including vincristine and vinblastine, represent ancient plant-based therapies widely used to treat cancers such as melanoma.6 Other natural compounds used in cancer treatment include phenolic compounds,7 cruciferous vegetables,8 benzopyrans,9 and Dillenia indica fruits.10C. roseus contains volatile compounds and phenolics, such as flavonol glycosides and caffeoylquinic acids, which defend the plant by combating reactive oxygen species.11C. roseus exhibits a wide range of medicinal qualities properties, including vasodilation, anti-allergic, anti-inflammatory, antimicrobial, antidiabetic, antithrombotic, cardioprotective,12 antioxidant, antifungal, antibacterial, and antiviral activities.13,14 Specific examples include: Methanol leaf extract, which promotes wound healing; vincamine and vindoline, which prevent ulceration; leaf juice, which possesses antiatherosclerotic properties; vinpocetine, which effectively treats Alzheimer’s disease; vincamine alkaloids, which provide neuroprotective and vasodilatory effects; and vinculin, which demonstrates hypoglycemic activity.15 In studies using Pheretima posthuma, C. roseus extracts have shown anthelmintic, anti-diarrheal, and hypotensive effects. Additionally, the plant demonstrates potential in the phytoremediation of radioactive cesium (Cs137), underscoring both its medical and environmental significance.16,17 Herbal plants, long used to treat infectious and chronic diseases, are considered safe, effective, and accessible alternatives to conventional medicines due to their rich phytochemical content.18 The dried leaves or whole C. roseus plants are boiled to prepare an oral extract widely used to treat diabetes in regions such as Northeast India, Mozambique, the Philippines, Australia, Thailand, Jamaica, and Vietnam.[19.20] This method is also employed as an alternative therapy for cancers like throat, stomach, and esophageal cancer, with similar practices observed in Kenya. In Tamil Nadu, India, C. roseus whole plant powder is mixed with cow’s milk and consumed orally to manage diabetes.21 In Limpopo Province, South Africa,22C. roseus root is dried, ground, and decocted to treat gonorrhea. Similarly, in the Mutirikwi area of Zimbabwe,23 the plant is used for stomach aches, and in the Venda region of South Africa, it is employed for urogenital infections.24C. roseus, a medicinal plant renowned for its diverse phytochemical profile and therapeutic potential, continues to garner attention. By synthesizing current knowledge, this review aimed to elucidate the plant’s chemical composition, pharmacological activities, and underlying mechanisms of action. Special emphasis is placed on its role in modern medicine, particularly its applications in cancer therapy, antimicrobial activities, and other health benefits attributed to its bioactive compounds. The rationale for this review stems from the increasing interest in natural products as sources of novel therapeutic agents and the growing demand for alternative and complementary medicines.
Research objectives and key issues
This review aimed to provide a detailed analysis of C. roseus by exploring its phytochemical composition, therapeutic potential, and mechanisms of action. It highlights the plant’s diverse phytochemicals, such as alkaloids, flavonoids, and phenolics, which contribute to its wide-ranging pharmacological activities. The therapeutic potential of C. roseus is examined across various domains, including its anticancer, antidiabetic, antimicrobial, neuroprotective, and other health-promoting properties. The review also delved into the biological mechanisms that underlie these therapeutic effects. Additionally, it discusses the plant’s traditional applications and their relevance in modern medicine, identifying key research gaps and emphasizing the need for clinical validation and further exploration of C. roseus for novel drug development.
Methodology
Databases Utilized: The literature for this review was sourced from reputable scientific databases, including PubMed, Scopus, and the Web of Science. These platforms were chosen for their extensive collection of peer-reviewed articles and relevance to pharmacological and phytochemical research.
Publication Timeline: Articles published between 1999 and 2024 were included to ensure the review incorporates both foundational studies and the most recent advancements in the study of C. roseus. This timeline reflects evolving research methodologies and contemporary relevance.
Inclusion Criteria: Articles published in English to maintain consistency and accessibility of information. Experimental studies, reviews, and meta-analyses focusing on the phytochemicals, pharmacological properties, or mechanisms of action of C. roseus. Studies with robust methodologies, clear outcomes, and peer-reviewed status.
Exclusion Criteria: Articles not published in English. Studies lacking peer review or employing outdated methodologies inconsistent with current scientific standards. Research focusing on unrelated species or using C. roseus in non-therapeutic contexts. Articles with insufficient data or unclear methodologies.
Botany of C. roseus
Catharanthus roseus (L.) Don, a significant evergreen herb in the Apocynaceae family, belongs to the kingdom Plantae, division Magnoliophyta, class Magnoliopsida, order Gentianales, and genus Catharanthus. The herbaceous C. roseus features glossy, oval-shaped, hairless green leaves arranged in opposite pairs on short petioles. The plant grows up to one meter tall, with leaves measuring 2.5–9 cm in length and 1–3.5 cm in width. Its flowers range in color from white to deep pink with a dark red center. They consist of a basal tube 2.5–3 cm long, an elongated corolla tube, a long pubescent ovary, and five petal-like lobes on the corolla spanning 2–5 cm in diameter. The calyx is short, the stigma is pentagonal, and the fruits consist of two follicles measuring 15–25 cm in length.25C. roseus contains nitrogen-rich alkaloids distinct from amino acids, peptides, purines, amino sugars, and antibiotics. Unlike compounds such as ephedrine, cathinone, and colchicine, these alkaloids lack nitrogen atoms within a ring system.26 Misra et al. reported that salinity stress (100 mM NaCl) stimulates the proliferation of indole alkaloids in the leaves and roots of C. roseus.27 Similarly, treating C. roseus cells with cadmium (0.05 to 0.4 mM) for 24–48 h enhanced the release of ajmalicine into the culture medium, particularly during the exponential growth phase.28 Additionally, UV-B light was found to increase alkaloid production in C. roseus hairy roots by activating protein kinases. Optimal pH levels in the culture medium elevated total nitrogen and phosphate levels, which in turn boosted protein and alkaloid yields. Nitrogen is critical for alkaloid synthesis, while phosphorus plays a supporting role in this process.29,30 Worldwide traditional utilization of C. roseus is summarized in Table 1.25,31–35
Worldwide traditional utilization of different parts of C. roseus
| Sl.no. | Plant parts | Preparation/extraction form | Mode of administration | Diseases | Country | References |
|---|---|---|---|---|---|---|
| 01. | Leaves | Dried and boiled with water | Oral intake | Menorrhagia, Diabetes | Australia | 31 |
| 02. | Root bark | Dried and boiled with water | Oral intake | Febrifuge | Australia | 31 |
| 03. | Whole plant | Dried and boiled with water | Oral intake | Diabetes | Brazil | 32 |
| 04. | Aerial portion | Dried and boiled with water | Oral intake | Menstrual regulators | China | 33 |
| 05. | Whole plant | Dried and boiled with water | Oral intake | Diabetes | England | 25 |
| 06. | Leaves | Dried leaf decocted | Oral intake | Diabetes | Europe | 33 |
| 07. | Whole plant | Dried and boiled with water | Oral intake | Anti-galactagogue | France | 25 |
| 08. | Whole plant | Dried and boiled with water | Oral intake | Cancer, Hodgkin’s disease, menorrhagia | India | 33 |
| 09. | Whole plant | Dried and boiled with water | Oral intake | Diabetes | Pakistan | 34 |
| 10. | Leafy stem | Boiled with water | Oral intake | Diabetes | West indies | 25 |
| 11. | Whole plant | Powdered and mixed with cow’s milk | Oral intake | Diabetes | India | 35 |
| 12. | Root | Root air dried, ground and decocted | Oral intake | Urogenital infections | South Africa | 35 |
| 13. | Whole plant | Boiled with water | Oral intake | Diabetes, Hypertension, Dysentery, Cancer | Vietnam | 35 |
Health-promoting effects of C. roseus
C. roseus is a renowned medicinal plant rich in diverse phytochemicals, exhibiting significant antioxidant, antibacterial, antifungal, antidiabetic, and anticancer properties.36 Its enduring importance lies in its potential as a source of novel therapeutic agents, as evidenced by the discovery of new compounds from C. roseus with promising applications in the treatment of diabetes and cancer.36 The various biological activities of C. roseus are summarized in Table 2.37–45
Different biological activities of C. roseus
| Sl.no. | Activities | Plant part | Plant extract | Bioactive compound | Treatment | Results | References |
|---|---|---|---|---|---|---|---|
| 01. | Anti-cancer | Stem leaves | Methanolic | Vinblastine, Vincristine | Neoplasms, choriocarcinoma Hodgkins disease | Inhibition concentrations: 10 ppm, 100 ppm, 1,000 ppm | 37 |
| 02. | Anti-diabetic | Leaves flower | Ethanolic | Vinculin | Streptozotocin induced model diabetic rats | Blood sugar Hypoglycemic effect | 38 |
| 03. | Anti-microbial | Leaves | Ethanolic | Indole alkaloids phenolic compounds | Bacterial cultures | Antimicrobial agent | 39 |
| 04. | Anti-oxidant | Roots | Ethanolic extract | – | DPPH assay superoxide radical scavenging | Antioxidant | 40 |
| 05. | Anthelmintic | Ethanolic | Helminthic infections | Growth | 41 | ||
| 06. | Anti-diarrheal | Leaves | Ethanolic | Flavonoids saponins | Anti-diarrheal | Gastrointestinal transit | 42 |
| 07. | Wound healing | Leaves | Ethanolic | Flavonoids triterpenoids | Incision wound model | Wound healing | 43 |
| 08. | Hypoglycemic activites | Leaves | Ethanolic | Vindoline, Vindolicine, Vindolin | PTP-1B inhibition, ORAC, DPPH, pNPP | Hypoglycemic antioxidant | 44 |
| 09. | Hypolipidemic effect | Leaves | Juice | Vinpocetine | High diet | No significant change in lipid profile and body weight | 45 |
Anti-neoplastic action
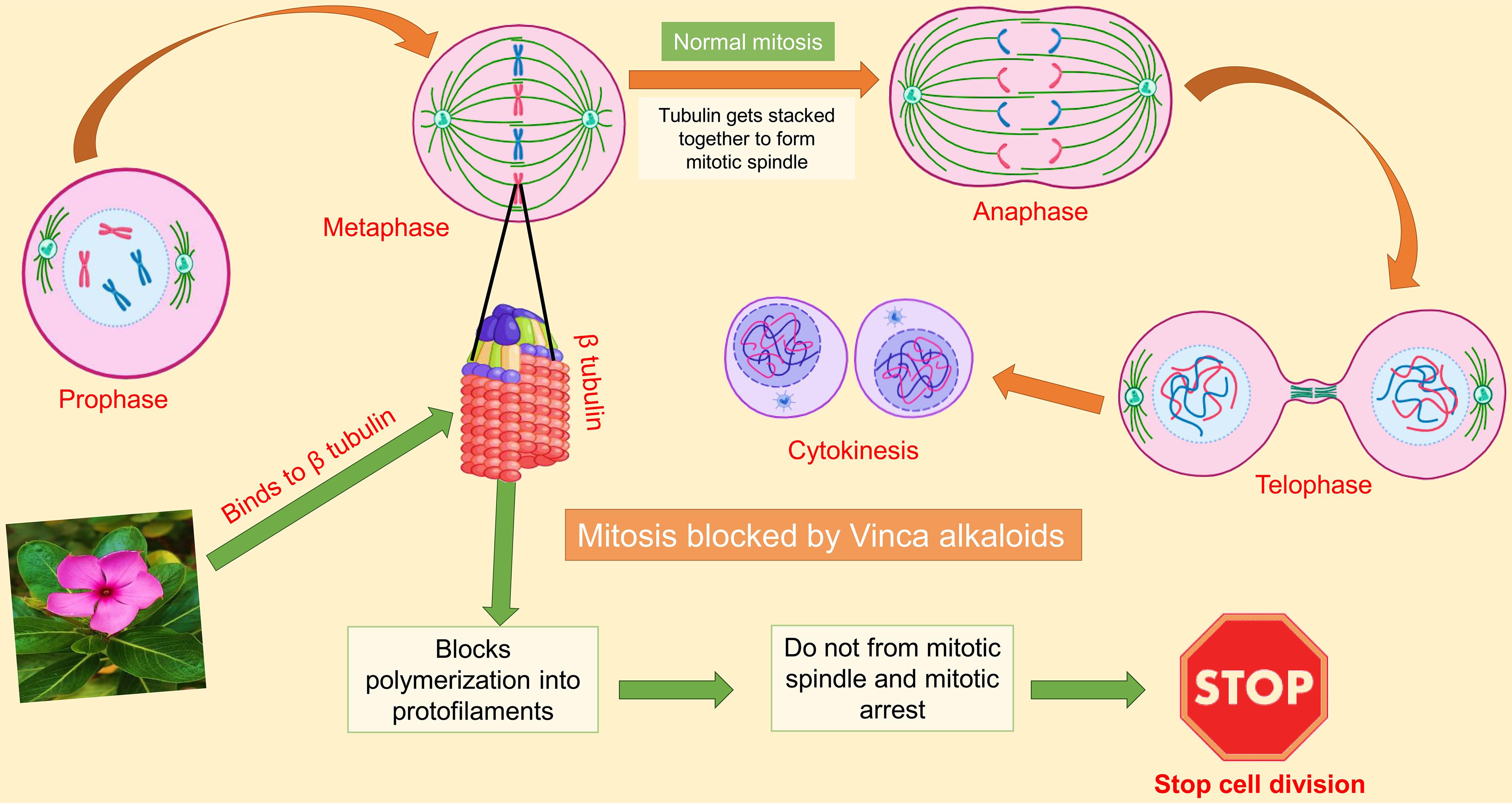
Cancer, a leading cause of mortality worldwide, is characterized by the uncontrolled proliferation of abnormal cells due to genetic mutations. Chemotherapy, often combined with radiotherapy, is a primary treatment modality; however, these treatments can damage healthy cells, leading to adverse effects.46 Certain plant-derived secondary metabolites hold promise for cancer prevention and treatment by modulating biological processes such as signaling pathways and cell proliferation. Vinblastine and vincristine gained prominence in the 1990s for their remarkable effectiveness in targeting and eliminating cancer cells.35 Jordan’s study underscores the significance of plant-derived Vinca alkaloids in cancer treatment, particularly their ability to target α/β-tubulin proteins essential for microtubule formation and cell division. By disrupting microtubule function, these compounds effectively inhibit cancer cell growth and induce apoptosis, facilitating the elimination of cancerous cells, as illustrated in Figure 1.47
In this process, tubulin attaches to the mitotic spindle, and normal cell division happens. However, in the second part, Vinca alkaloids and their derivatives inhibit the cancer cell cycle during mitosis by depolymerizing the microtubules and binding at the surface between two tubulin heterodimers next to the exchangeable GTP binding site, arresting cell division. GTP, Guanosine triphosphate.
Vinblastine, a vinca alkaloid, is widely used to treat various cancers, including neuroblastoma, lymphosarcoma, lung and breast cancers, Hodgkin’s disease, and lymphocytic leukemia. Vincristine, meanwhile, is primarily used for specific leukemias and lymphomas.48 Compounds like catharoseumine from C. roseus have demonstrated potential in laboratory tests to inhibit the growth of breast cancer and leukemia cells.49 Important Vinca alkaloids, such as vindesine, vinblastine, vinorelbine, and vincristine, are approved for cancer treatment in the United States. Additionally, vinflunine, the first fluorinated microtubule inhibitor, is used in Europe to treat urothelial carcinoma.50 Vinflunine disrupts microtubule formation and has shown potent effects against cancers such as leukemia, breast, lung, and Hodgkin’s disease, often complementing other treatments for comprehensive cancer care.51 Anhydrovinblastine induces cell death in leukemia, carcinoma, and lung cancer cells, while vinorelbine effectively treats non-small cell lung cancer, advanced breast cancer, and Hodgkin’s lymphoma.52 Vincristine is favored for its lower risk of nerve damage and bone marrow suppression, whereas vinblastine is often combined with other chemotherapy drugs to prevent resistance.53 Vinca alkaloids activate pathways such as nuclear factor kappa B (NF-κB) and JNK to induce apoptosis in cancer cells, both in vitro and in vivo.54 These pathways damage cancer cell DNA and impair mitochondrial function, with NF-κB influencing tumor progression and JNK responding to cellular stressors to regulate cellular processes. Additionally, JNK modifies the c-Jun protein, further contributing to cellular regulation.55 Details about various alkaloids used as chemotherapeutic agents are presented in Table 3.25,56–59
Details of various alkaloids that are used as chemotherapeutic agents
| S.no. | Indole alkaloid | Trade (Market name) | Treatment | Type of alkaloids | Pharmacological mechanisms | Side effects | In-vitro/In-vivo testing | References |
|---|---|---|---|---|---|---|---|---|
| 01. | Vinblastine sulphate | Velban | Hodgkin’s disease, Lymphosarcoma, Neuroblastoma, Carcinoma of breast, Lung cancer, Head and neck cancer, Testicular cancer | Anti-mitotic | Clings to tubulin, prevents microtubules from developing | Bone marrow toxicity, Gastrointestinal toxicity, Potent vesicant, Extravasation injury | Chronic lymphocytic leukemia (CLL) | 25 |
| Oncovin | Neuroblastoma, Wilkins’s tumor, Hodgkin’s disease, Breast cancer, Lung cancer, Head and neck cancer, Testicular cancer | Anti-mitotic | Clings to tubulin, prevents microtubules from developing, Binds to tubulin dimer, prevents microtubule structures from forming | Bone marrow toxicity, Gastrointestinal toxicity, Potent vesicant, Extravasation injury, Peripheral neuropathy, Hyponatremia, Constipation, Paralysis, Spinal nerve demyelination, Lung spasm | HL60 human acute promyelocytic leukemia cells, K526vhuman chronic myelogenous leukemia cells, NHK 3025 and EA.hy926 human umbilical vein cells, B-cell lymphoma cell line | 56 | ||
| 02. | Vinblastine, Vincristine | Vinflunine | Transitional cell carcinoma, Breast cancer | Anti-mitotic | Transition from metaphase to anaphase, preventing cancer cells from entering mitosis | Hair loss, Weariness, Overall sensation of weakness | Human tumour cell lines (ECV304, MCF-7, H292, and CAL-27) | 57 |
| 03. | Vindesine | Eldisine and Fildesin | Melanoma, Lung cancer, Uterine malignancies | Anti-mitotic | Mitotic arrest in metaphase. | Spinal nerve demyelination, Hyponatremia, Constipation, Hair loss, Breathing problems, Lung spasm | K562 and a T-cell leukaemia-derived cell line, MOLT-4 | 58 |
| 05. | Vinorelbine | Navelbine, Binorel, Biovelbin, Eunades, Flonorbin, Neoben, Relbovin, Vinelbine, Vinorelbel, Vinotec | Breast cancer, Non-small cell lung cancer | Anti-mitotic | Binding to microtubular proteins in the mitotic spindle, thereby preventing cell division during metaphase | Inflammation of veins, Constipation, Poor resistance to infection, Bleeding, Anemia | NSCLC cell lines, A549, Calu-6, and H1792 | 59 |
Antidiabetic action
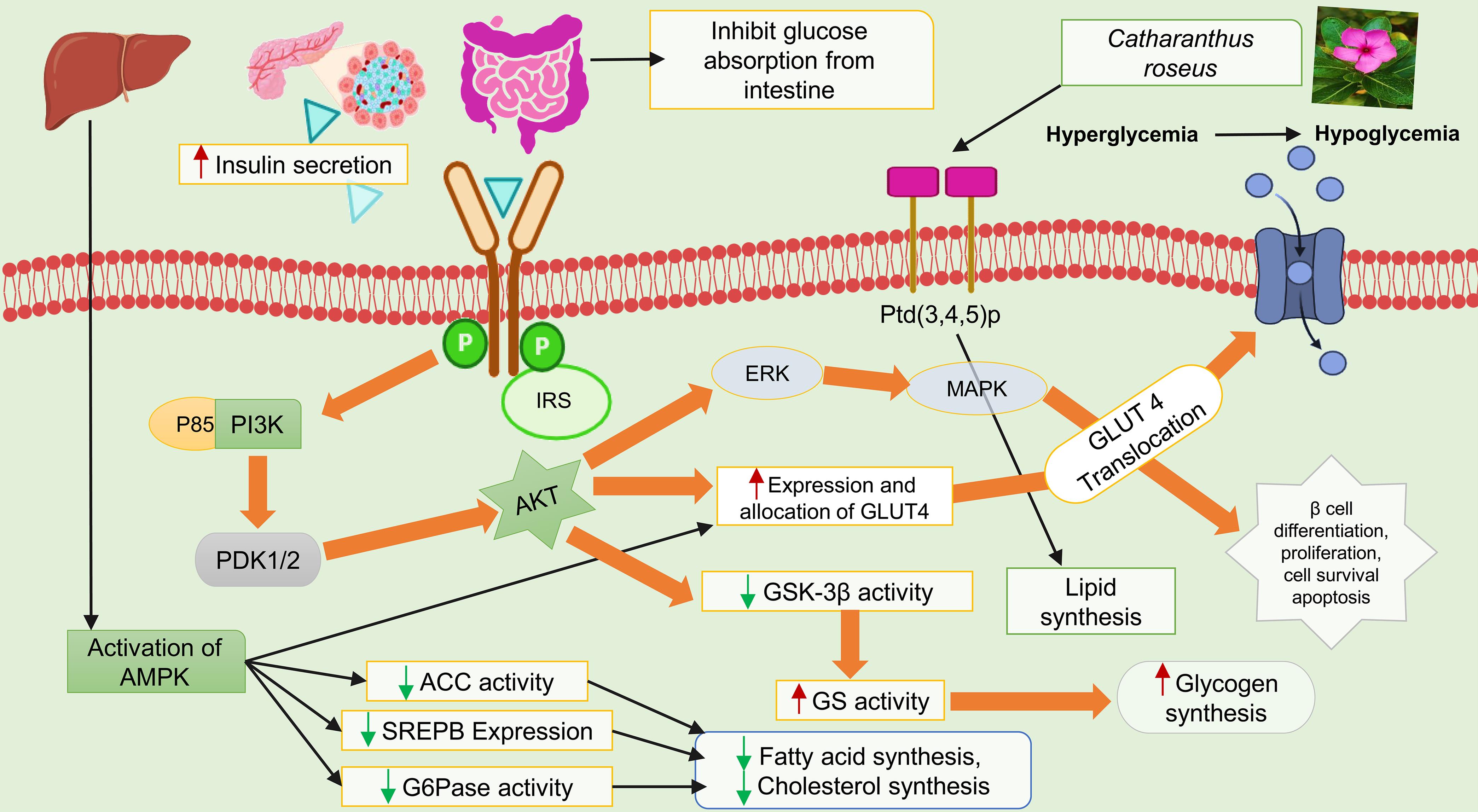
Diabetes triggers oxidative stress, leading to DNA, protein, and lipid damage while impairing antioxidant defenses. This contributes to complications like atherosclerosis due to excessive production of reactive oxygen species.60 Research shows a strong link between oxidative stress markers, such as lipid peroxidation, and diabetes, with byproducts like malonaldehyde and free radicals worsening cellular damage and vascular health.61 The leaf extract of C. roseus contains alkaloids such as vindoline, vindolicine, and others. At a concentration of 25 µ/mL, vindoline and vindolicine showed no negative effects on pancreatic TC6 cells, while vindolicine demonstrated high antioxidant activity in oxygen radical absorbance capacity and 2,2-diphenyl-1-picryl hydroxyl assays.62 A methanolic extract of the whole plant significantly improved lipid profiles, pancreatic β-cell function, and body weight in diabetic rats, showing a strong anti-hyperglycemic effect.63 Tiong et al. reported that alkaloids such as vindoline and vindolicine from C. roseus leaves exhibit hypoglycemic properties by inhibiting PTP-1B, an enzyme that disrupts insulin signaling, and by enhancing glucose uptake in C2C12 and β-TC6 cells.64 Oral administration of C. roseus flower aqueous extract in diabetic rats reduced blood glucose, improved lipid profiles, and delayed body weight loss at doses of 250, 350, and 450 mg/kg for 30 days.65 The hypoglycemic effect of C. roseus is illustrated in Figure 2.
By activating AMPK, the expression of glucose transporter-4 is enhanced, leading to its translocation to the cell membrane and increased glucose uptake, showing a hypoglycemic effect. It also increases beta cell proliferation in the pancreas, which boosts insulin secretion and glucose clearance. AMPK, AMP-activated protein kinase.
Neuroprotective action
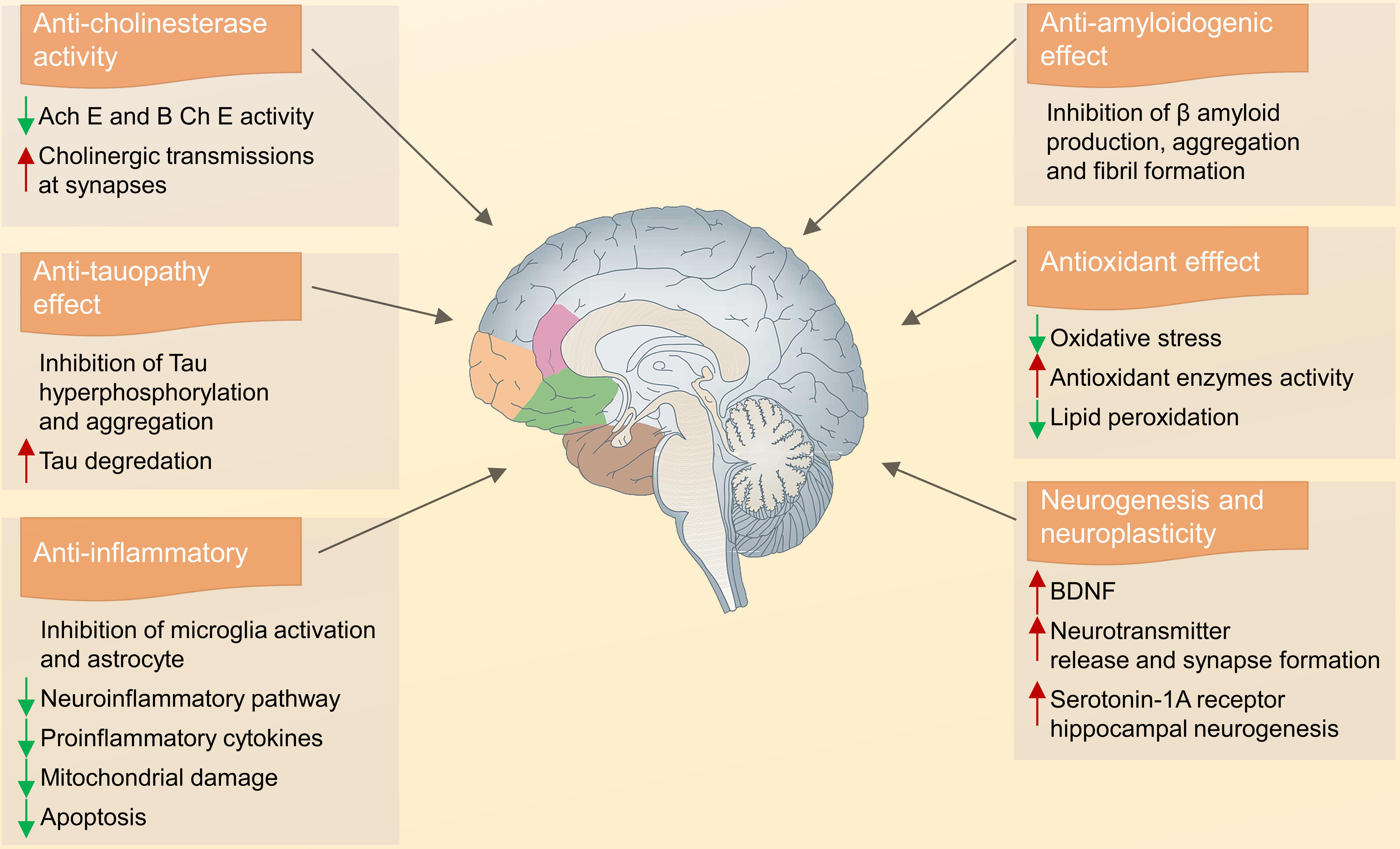
Alzheimer’s disease (AD) causes significant damage to cholinergic neurons in the neocortex and hippocampus, leading to reduced glutamate levels and symptoms such as memory loss, personality changes, and emotional instability.66 The most effective treatment for AD involves inhibiting acetylcholinesterase (AchE) to enhance cholinergic neurotransmission and increase acetylcholine levels.67 Aqueous extracts from the leaf, stem, and root of C. roseus have been shown to effectively inhibit AchE in an in-vitro micro-assay.68 Clinical trials for early-stage dementia and stroke identified a safe dose of up to 60 mg/day with no significant adverse effects. Additionally, serpentine, an alkaloid from C. roseus, demonstrated a low AchE IC50 (0.775 µM), improving cerebral blood flow, metabolism, and glucose absorption, potentially mitigating hypoxia and ischemia effects.69 Vinpocetine, a nootropic alkaloid, enhances memory and learning, reduces the effects of trauma, ischemia, and stroke on the brain, and increases neuroprotective gene expression and neural plasticity.70 It also exhibits potent anti-inflammatory effects by modulating the NF-κB pathway, inhibiting I-κB kinase, and reducing cerebral infarction, making it a common treatment for ischemic stroke and cognitive deficits.71 The various neuroprotective actions of C. roseus are shown in Figure 3.
It also inhibits the hyperphosphorylation of tau protein, increases the levels of BDNF and serotonin, and decreases lipid peroxidation. It also has anti-inflammatory effects and inhibits amyloid β production. BDNF, Brain-Derived Neurotrophic Factor
Vascular disruption
Destabilization of tubulin, leading to vascular disruption, is linked to antiangiogenic effects. Vinblastine inhibits endothelial cell functions, destabilizes microtubules, and induces apoptosis, effectively blocking tumor blood vessels and demonstrating its potential as a cancer treatment.72 Catharoseumine, isolated from C. roseus, has shown cytotoxic effects against human promyelocytic leukemia HL-60 cell lines, indicating its potential to reduce cancer cell proliferation.73 Vinca alkaloids disrupt cell division by interfering with the cell cycle, causing lethal effects on cancer cells and non-cancerous cells alike, making them promising candidates for future cancer therapies, especially in combination with other anti-cancer drugs.74
Antihyperlipidemic action
C. roseus exhibits antihyperlipidemic effects by reducing harmful blood lipids like triglycerides, low-density lipoproteins, cholesterol, and total cholesterol, with notable reductions at higher doses in experimental models. Additionally, it shows promise in lowering blood pressure, further enhancing its cardiovascular benefits.75
Antimicrobial action
C. roseus demonstrates potent antibacterial properties, with extracts from its leaves, stems, flowers, and roots effectively combating a variety of bacteria, fungi, and malaria-causing parasites. Compounds such as phenolics, flavonoids, and catharoseumine exhibit fungi-toxic effects against crop disease fungi and antibacterial activity against pathogens like Pseudomonas aeruginosa and Staphylococcus aureus.76 Additionally, C. roseus contains catharoseumine, which targets the parasite Falcipain-2, responsible for malaria.49 Saponin-rich fractions from the plant’s stem and root show significant antifungal activity against Aspergillus niger and Candida albicans. Substances like catharoseumine inhibit the parasite causing human trypanosomiasis, while Catharanthus exhibits antiviral activity against the herpes simplex virus.77 These findings highlight the potential of C. roseus extracts as powerful antibacterial agents amid rising microbial resistance.76
Wound healing action
Wound healing is a complex process that restores damaged tissues and cellular structures. It begins with the removal of damaged tissue during the fibroblastic stage and progresses through three phases: inflammatory, proliferative, and maturation. In the proliferative phase, collagen production, blood vessel formation (angiogenesis), and skin cell growth (epithelialization) occur following inflammation and blood clotting.78 The ethanolic extract of Vinca rosea has shown excellent therapeutic benefits during the maturation period of wound healing, which is characterized by wound constriction and scar formation. Studies show that this ethanolic extract increases collagen synthesis and significantly strengthens tissue in rats.79 At specific dosages, it exhibits potential in healing diabetic mice, with high levels of hydroxyproline indicating its effectiveness. Hydroxyproline is a crucial component and reliable marker of collagen synthesis, essential for strong and healthy tissue regeneration.80
Antioxidant action
Antioxidants protect the body from harmful free radicals generated during oxidation, which can lead to neurological issues and cancer. Vinca rosea is particularly potent due to the vitamin C, alkaloids, and tannins in its leaves, all of which help combat free radicals.64 With a higher phenolic content than other plants, C. roseus exhibits stronger antioxidant effects. Studies have shown that C. roseus extracts suppress the growth of cancer cells, demonstrating significant antioxidant capability.81C. roseus contains phytoconstituents like tannins, phenolics, and flavonoids, which contribute to its strong antioxidant activity, particularly at concentrations around 800 µg.82 The plant has the highest oxygen radical absorbance capacity among therapeutic herbs, with its flowers outperforming common antioxidants like L-ascorbic acid.83 Alkaloids such as vindoline and vindolicine effectively scavenge free radicals and prevent oxidative damage. Comparisons show that C. roseus exhibits higher antioxidant capacity than C. alba, particularly in ethanolic root extracts, while triaimefon may enhance alkaloid synthesis, further boosting its therapeutic potential.84 The different antioxidant properties of C. roseus are depicted in Figure 4.
Other clinical conditions
Vinpocetine, derived from C. roseus, holds significant therapeutic potential for neurological and cardiovascular diseases. A two-week study showed improved cerebral blood flow in ischemic stroke patients, with further research needed for acute stroke. It enhances cognitive and motor function in Alzheimer’s and age-related brain decline, improves memory, and reduces symptoms of sundowning syndrome. Vinpocetine also shows promise in treating tinnitus, Meniere’s disease, and epilepsy by reducing seizures and improving psychomotor performance. Additionally, C. roseus offers benefits such as anti-diarrheal, anti-fertility, and hypotensive properties, along with lipid-lowering effects in hypertension.
Chronic cerebral vascular ischemia
A study involving fifteen patients with chronic ischemic stroke showed that a two-week trial of vinpocetine significantly increased cerebral blood flow in the unaffected hemisphere. Using near-infrared spectroscopy and Doppler sonography, it was found that 10% of patients with chronic cerebrovascular disease, who received a specific vinpocetine infusion, exhibited improved perfusion in the middle cerebral artery.85
Acute ischemic stroke
Out of eight studies involving acute stroke patients (treated with vinpocetine within two weeks of the stroke), only one met the criteria for meta-analysis. In this trial, eight out of seventeen patients receiving vinpocetine and twelve out of sixteen receiving a placebo were classified as “dependent,” meaning they could not live independently. However, all patients remained alive three weeks after starting the IV vinpocetine treatment. This meta-analysis, based on in vitro studies and animal data, suggests that vinpocetine holds potential for treating acute stroke, though further well-designed studies are needed.
Dwindling senile cerebral
The researchers employed various psychological evaluation scales alongside assessments of physical symptoms, such as speech and movement abilities, muscle coordination and strength, and sensory perception skills, to demonstrate the remarkable effectiveness of vinpocetine on both motor and cognitive functions.86
Alzheimer’s disease
Vinpocetine significantly improves short-term memory loss, age-related dementias, AD, vascular dementia, and prolonged memory impairment. It enhances memory and cognitive function.87–89
Sundowning syndrome
AD often causes behavioral symptoms that are most pronounced in the evening, a phenomenon known as “Sundowning Syndrome.” Vinpocetine is commonly prescribed to help prevent these behavioral dysfunctions in affected patients.87,90
Tinnitus, Meniere’s disease, and visual issues
The management of auditory trauma associated with eventual hearing loss and tinnitus has involved the consumption of vinpocetine.91 Treatment for Meniere’s disease and common lesions secondary to arteriosclerosis with vinpocetine has proven beneficial.92
Epilepsy
Vinpocetine (15–45 mg/day) significantly reduces the frequency of attacks, especially when combined with absences and generalized tonic-clonic seizures. In addition to its anticonvulsant effects, vinpocetine also enhances psychomotor function and helps reduce intracranial hypertension.
Miscellaneous action
C. roseus leaf extract has been shown to possess hypotensive and lipid-lowering properties, as demonstrated in rats with adrenaline-induced hypertension.75 Ajmalcine in C. roseus is also considered a hypotensive agent and acts as an antagonist of the alpha-adrenergic receptor.93 Employing castor oil as a test, and loperamide and atropine sulfate as the standard drugs, the in vivo anti-diarrheal activity of C. roseus ethanolic leaf extract was assessed in Wistar rats.94 There is a dose-dependent reduction of castor oil-induced diarrhea at 200 and 500 mg/kg of C. roseus extract.95 The decline of dopamine release that tends to happen with aging can be mitigated by vinpocetine. The role of vinpocetine in pathological cardiac remodeling, fibrosis, and cardiac hypertrophy is being investigated in recent in vivo and in vitro studies. Vinpocetine suppressed the hypertrophic growth of adult mouse cardiomyocytes and the activation of cardiac fibroblasts in vitro, possibly via a phosphodiesterase-1-dependent mechanism. The significant decrease in glucose and fructose levels in reproductive tissues, the hyalinization of tubules, widespread testicular necrosis, and Sertoli cells-only syndrome following oral administration of C. roseus Linn. leaf extract highlights the extract’s anti-fertility attributes.96 The petroleum ether extract of C. roseus leaves decreased the estrogenic effect attained in uterine weight when given to female albino mice paired with estradiol, implying that this combination is effective in preventing pregnancy.97 The effectiveness of C. roseus solvent extract against the larvae of the gram pod borer Helicoverpa armigera was verified biologically, demonstrating the insecticidal properties of C. roseus.98 Various alkaloids obtained from C. roseus are described with their structures and mechanisms of action in disease in Table 4.
Details about various alkaloids obtained from C. roseus with their chemical structure and mechanism of action
| S.no. | Chemical compound | Chemical structure | Mechanism of action in particular diseases | References |
|---|---|---|---|---|
| 01 | Vinblastine | 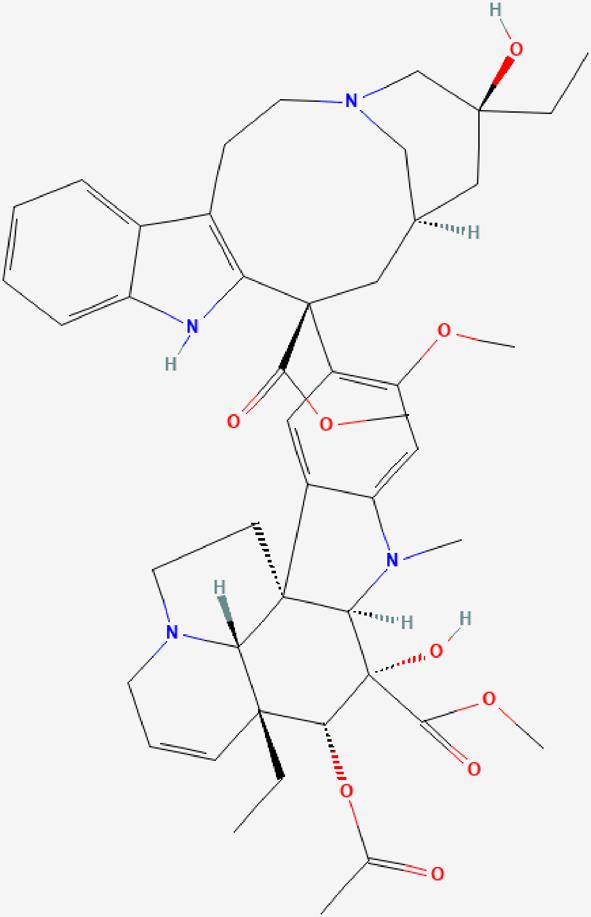 | It binds to microtubular proteins in the mitotic spindle, thereby preventing cell division during the metaphase-Antineoplastic effect. | https://pubchem.ncbi.nlm.nih.gov/compound/Vinblastine |
| 02 | Vincristine | 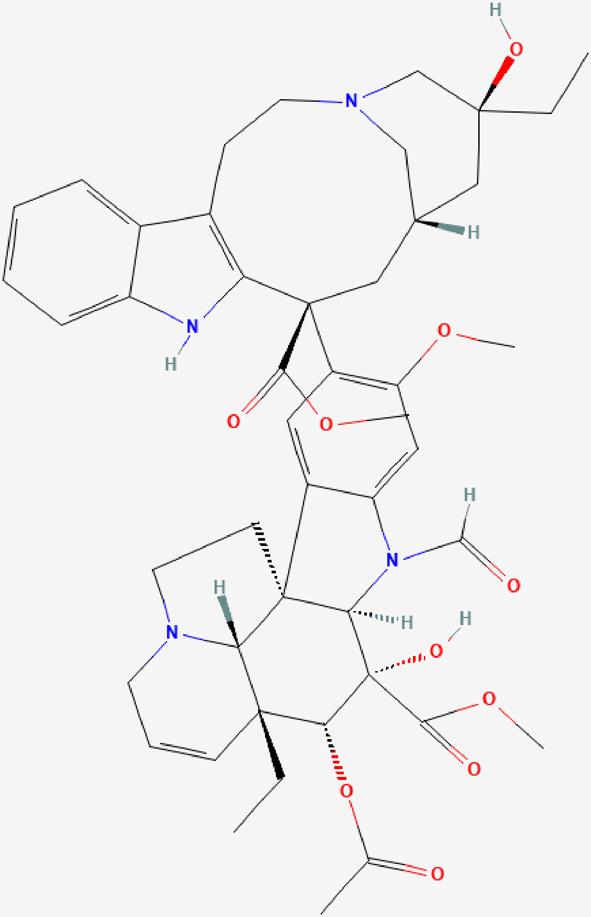 | Inhibition of microtubule formation in mitotic spindle- Antineoplastic effect | https://pubchem.ncbi.nlm.nih.gov/compound/Vincristine |
| 03 | Vindoline | 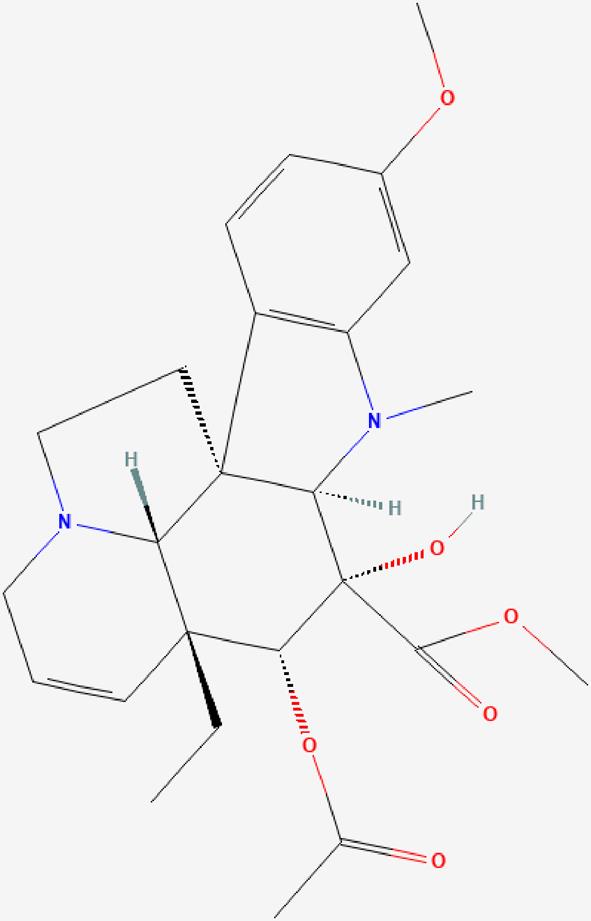 | Stimulating insulin secretion and inhibiting certain enzymes related to carbohydrate metabolism-Antidiabetic effect. | https://pubchem.ncbi.nlm.nih.gov/compound/Vindolin |
| 04 | Vindolicine | 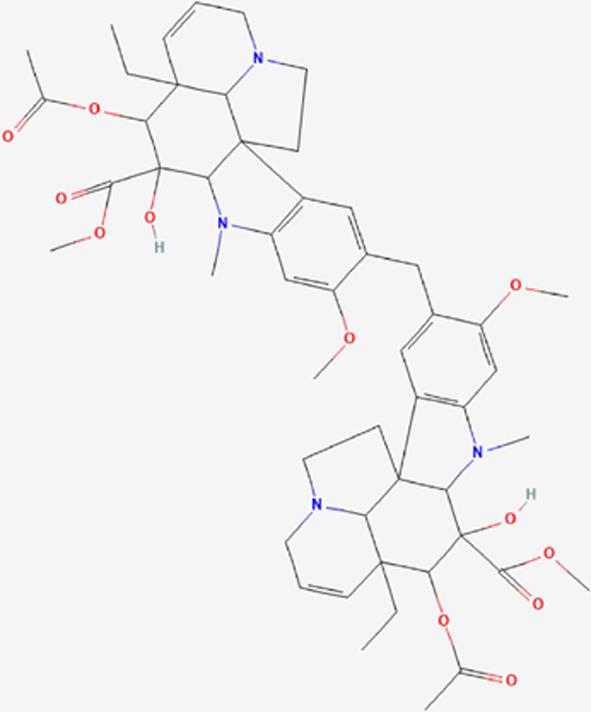 | Stimulating insulin secretion and reducing blood sugar levels -Antidiabetic effect (Diabetes mellitus-2) | https://pubchem.ncbi.nlm.nih.gov/compound/Vindolicine |
| 05 | Vinpocetine | 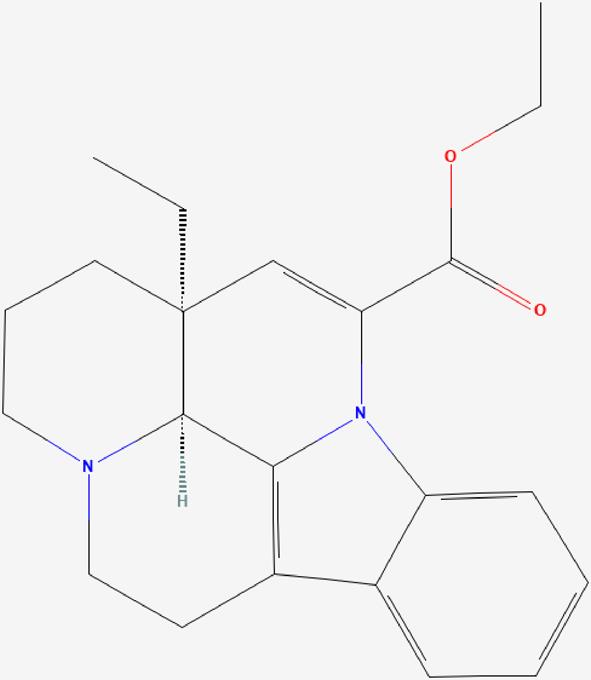 | Inhibits PDE1 activity and improves cerebral blood flow by elevating cGMP and cAMP, increasing mitochondrial function, and improving glucose and oxygen utilization by the brain. Vinpocetine helps improve spatial memory in rats by preventing neuronal damage and favorably modulating cholinergic function. | https://pubchem.ncbi.nlm.nih.gov/compound/Vinpocetine |
| 06 | Vindesine | 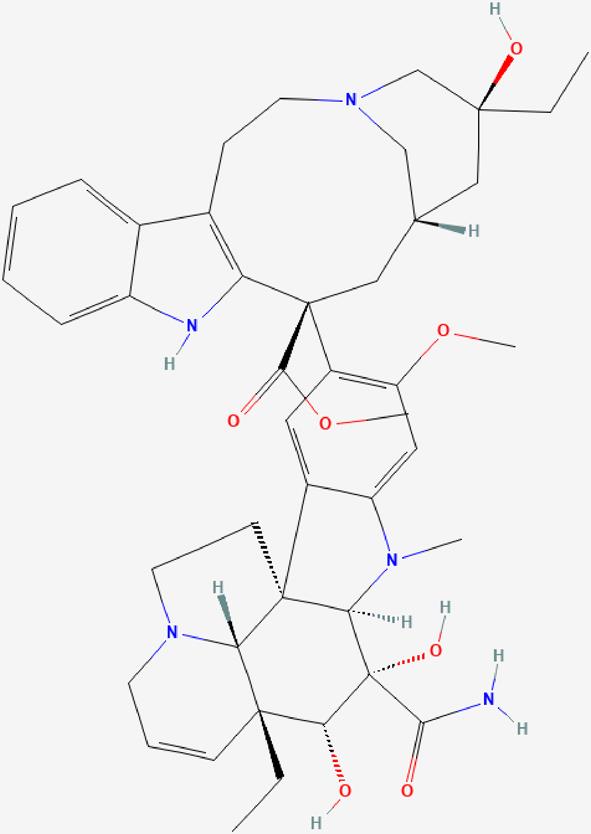 | Arrest of cells in metaphase mitosis-Antineoplastic effect. | https://pubchem.ncbi.nlm.nih.gov/compound/Vindesine |
| 07 | Vinorelbine | 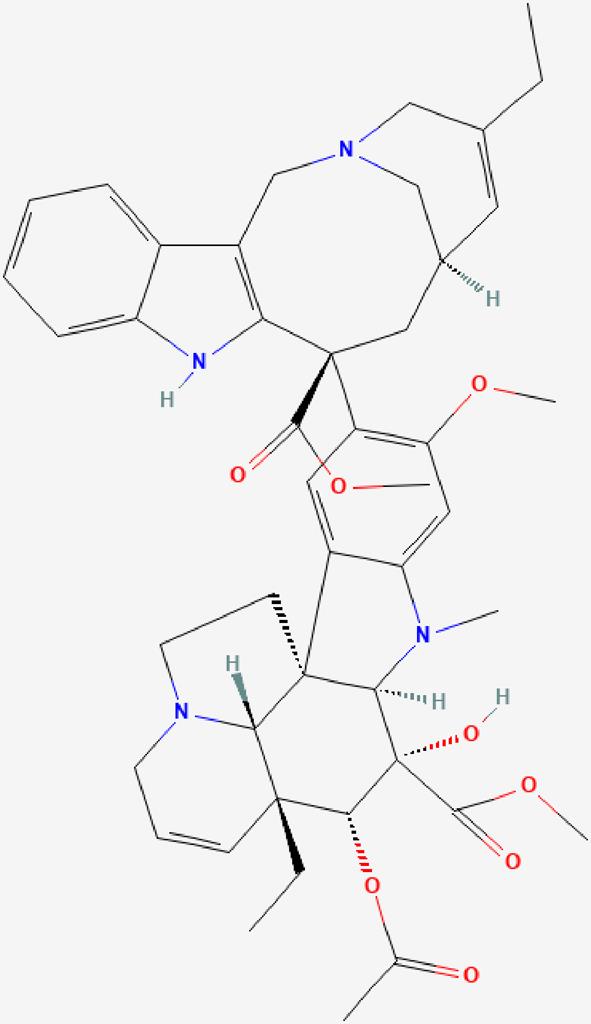 | It acts by binding to microtubular proteins in the mitotic spindle, thereby preventing cell division during metaphase- Antineoplastic effect. | https://pubchem.ncbi.nlm.nih.gov/compound/Vinorelbine |
| 08 | Vinflunine | 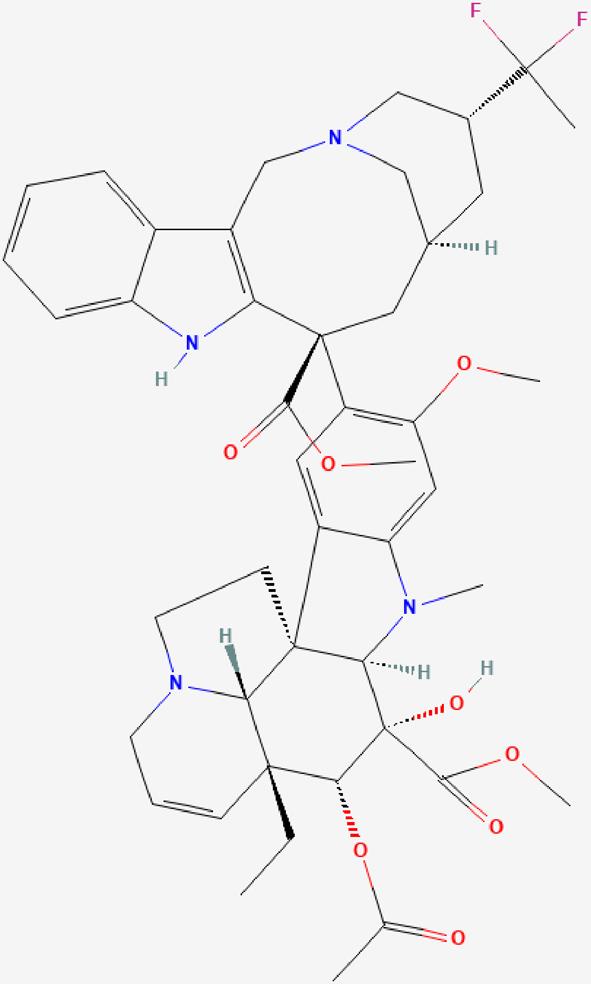 | Vinflunine is a microtubule inhibitor that binds to tubulin at or near the vinca binding sites to inhibit its polymerization into microtubules during cell proliferation-Antineoplastic effect. | https://pubchem.ncbi.nlm.nih.gov/compound/10629256 |
Toxicological profile
Due to psychological factors, intravenous (i.v.) administration is preferred for Vinca alkaloids such as vincristine and vinblastine; however, there are several potential side effects, including hypotension, renal impairment, nausea, and neurotoxicity.99–101 Vinorelbine is notable for being the first Vinca alkaloid to show effectiveness when taken orally, whereas vincristine, its equivalent, is more well-known for its potential to cause peripheral neurotoxicity.102 Neutropenia, leucopenia, and granulocytopenia are common toxicities associated with Vinca alkaloids, although vinorelbine is generally well-tolerated. Pregnant or breastfeeding women should exercise caution, and immunosuppressive effects necessitate avoiding vaccines during treatment. Vinpocetine, another Vinca derivative, offers promise for treating ischemia with relatively mild side effects such as headache and nausea. Patients receiving Vinca alkaloids should inform their clinicians about all concurrent medications and pre-existing conditions, as these can affect drug accumulation, cytotoxicity, and overall treatment safety. It is crucial to disclose any additional prescriptions or underlying health issues, including gout, kidney stones, viral infections like chickenpox or herpes zoster, liver disease, and nerve or muscle disorders.103
Future prospects
As more studies reveal C. roseus’ potential for therapeutic use, the plant’s future in conventional medicine looks promising. C. roseus, with its wide range of bioactive chemicals, has the potential to become a mainstay in integrative medicine, combining traditional methods with cutting-edge scientific understanding. Future research will probably concentrate on refining the extraction and formulation processes to improve the safety and efficacy profiles of the active ingredients. Advancements in biotechnological culture and processing may enable the large-scale manufacturing of C. roseus-based medications, increasing their global accessibility. Furthermore, as traditional medicine gains wider recognition, C. roseus may play a significant role in treating difficult and chronic illnesses, offering innovative cures for diseases that are resistant to existing treatments. Further investigation of its pharmacological properties and interactions will be crucial in establishing new therapeutic uses and ensuring the safe incorporation of this ancient remedy into modern medical practices.
Limitations
This review highlights significant findings on C. roseus, but several limitations remain. There is a lack of robust clinical trials to validate its therapeutic effects and long-term safety in humans. Attention has been given only to well-known compounds such as vincristine and vinblastine leaves, while emerging phytochemicals have been less explored. Additionally, variations in phytochemical composition due to geographical and environmental factors are not extensively addressed. Mechanistic insights into certain pharmacological activities remain incomplete, and the exclusion of non-English studies may introduce language bias. These limitations underscore the need for further research to bridge these gaps and enhance the understanding of this versatile plant.
Conclusions
C. roseus, renowned for its rich phytochemical composition, holds significant promise in addressing a range of therapeutic areas, including anticancer, antidiabetic, antioxidant, antimicrobial, and antihypertensive treatments. Key bioactive compounds, such as vinca alkaloids, phenolics, and indole alkaloids, have demonstrated remarkable potential in preclinical and clinical studies. In cancer treatment, compounds like vincristine and vinblastine remain indispensable, with emerging derivatives like vinflunine expanding therapeutic options for drug-resistant tumors. Furthermore, the plant’s bioactive constituents offer innovative strategies to overcome multidrug resistance, a critical challenge in oncology. Beyond oncology, the leaf extracts of C. roseus show promise in managing hypertension and hyperlipidemia, potentially complementing existing therapies for cardiovascular conditions. The cultivation of C. roseus in bioreactors following cGMP standards ensures scalable production of its pharmaceutical compounds. Additionally, its role in neurodegenerative research and other advanced pharmacological studies highlights its versatility and untapped potential in modern medicine. Despite its established benefits, further research is essential to validate these therapeutic applications and explore novel compounds to enhance drug development. By continuing to investigate its phytochemical and pharmacological properties, C. roseus could serve as a cornerstone for innovative pharmaceutical advancements.
Declarations
Acknowledgement
Special thanks to Shri. Parveen Garg, Chairman, ISFCP, for providing an excellent research platform. This work wouldn’t have been possible without their collective influence.
Funding
None.
Conflict of interest
The authors declare no conflict of interest.
Authors’ contributions
Conceptualization, review, editing, visualization (KRA), writing – review & editing the manuscript (AK, KM). All authors made significant contributions to this study and approved the final manuscript.

 Author information
Author information