Introduction
Aging has been proposed to involve multiple, progressive functional changes in cells, organs, tissues, and entire organisms as they grow older due to intrinsic (genetic) and extrinsic (epigenetic/environmental) factors.1–7 Aging is also a process that involves environmental, psychological, physiological, behavioral, and other biological changes.8 These changes can result in age-related multiple morbidities.9,10
During aging, the ability to maintain homeostasis and adapt to changing conditions is reduced, and susceptibilities to stress, injury, and disease are increased. It has been proposed that the elderly are more susceptible to age-related morbidities due to progressive functional declines in tissue and organ functions and a reduced ability to adapt physiologically.3,11–16
At the mechanistic level, aging has been linked to the progressive accumulation of cellular and molecular damage and to changes in various interlinked mechanisms that include damage to lipids, proteins, and nucleic acids, resulting in membrane and cell dysfunction and genomic instability; epigenetic changes; chronic inflammation; fibrosis; telomere attrition; stem cell exhaustion; ion and metabolic shifts; changes in metabolite generation; loss of proteostasis; disturbances in signal pathways and neurotransmitters; accumulation of misfolded or aggregated proteins; deregulated nutrient sensing; and other changes that reinforce an increase in general cellular dysfunction.1,3–5,12,13,17–24
During the process of aging and in essentially all chronic and acute illnesses, intracellular and cellular membranes, as well as other cellular components, are key constituents in positive feedback cycles involving damage, and they are modified, in part, by exposure to surplus free radical oxidants and other forms of impairment.12,21,25–30 Although damage to cellular structures has been purported to be an important event contributing to the aging process,25–29 it is now accepted that free radical oxidants, such as reactive oxygen and nitrogen species (ROS/RNS), are not the sole basis of aging or diseases promoted by aging.30–33 However, the production of excess ROS/RNS and other free radical oxidants, along with the cellular damage resulting from their reactions, is thought to play an important role, in addition to other mechanisms, in age-related changes and the initiation and progression of various chronic diseases.1,5,16,17–22,25–28,31,33–35
Cellular membranes
Among the important cellular components modified with aging and disease are the various cellular membranes involved in compartmentalizing cells and cell cytoplasm and separating various chemical, enzymatic, and signaling pathways within cells. Organelles like the nucleus, mitochondria, endoplasmic reticulum, endosomes, and other intracellular structures are bounded by characteristic membranes composed primarily of lipid and protein components. How these membrane components are organized into various functional cellular barriers has been under intense investigation for the last six decades.36–41
Cellular membranes in aqueous solutions are macromolecular structures held together predominantly by hydrophobic and van der Waals forces, as well as to a minor extent by ionic connections and a few covalent bonds. At nanometer distances, these forces and interactions result in the establishment of active membrane barriers consisting of amphipathic lipids, glycoproteins, and proteins that associate into predominantly non-covalently linked molecular assemblies possessing diverse degrees of lateral and rotational mobility.36–42 The basic barriers formed by biological membranes are built on a lipid bilayer matrix composed primarily of glycerophospholipids (GPL).43,44
From early experiments in the last century, three competing models for basic cellular membrane organization were proposed. Initially, Danielli and Davson suggested that cell membranes were composed of phospholipid bilayers interacting with compressed or flat-structured proteins (predominantly beta-sheet structured) through the proteins’ charged amino acids and the hydrophilic headgroups of the membrane GPL.45 Robertson used transmission electron microscopy to visualize this structure as a tri-molecular layered assembly of molecules.46 This depiction of a cell membrane was offered as proof of various cellular membranes being trimolecular layers or protein/phospholipid/protein sheets (Trilayer or Unit Membrane). A completely different type of cell membrane organization was presented by Benson and, separately, by Vanderkooi and Green, involving monolayers of repeating subunits of lipoproteins that did not incorporate a phospholipid bilayer into their structure (Lipoprotein Subunit/Protein Crystal Membrane).47,48 However, neither of these models was consistent with available data.36,37 In 1972, the Fluid–Mosaic Membrane (FMM) model was introduced as a general explanation for the structure and dynamics of cellular membranes. This model was based on a lipid bilayer with membrane glycoproteins and proteins intercalated into the lipid bilayer to varying degrees.36 Since its introduction, the FMM model has been considered the most suitable representation of cellular membrane structure and dynamics at the nanometer level compared to other models.39–41 (See Fig. 1 of Nicolson and Ferreira for a comparison of the three main models for biological membranes.) This is introduced here because the repair and maintenance of cellular membranes are critical to maintaining health and longevity and preventing positive feedback cycles generated during aging.17,18,20–22
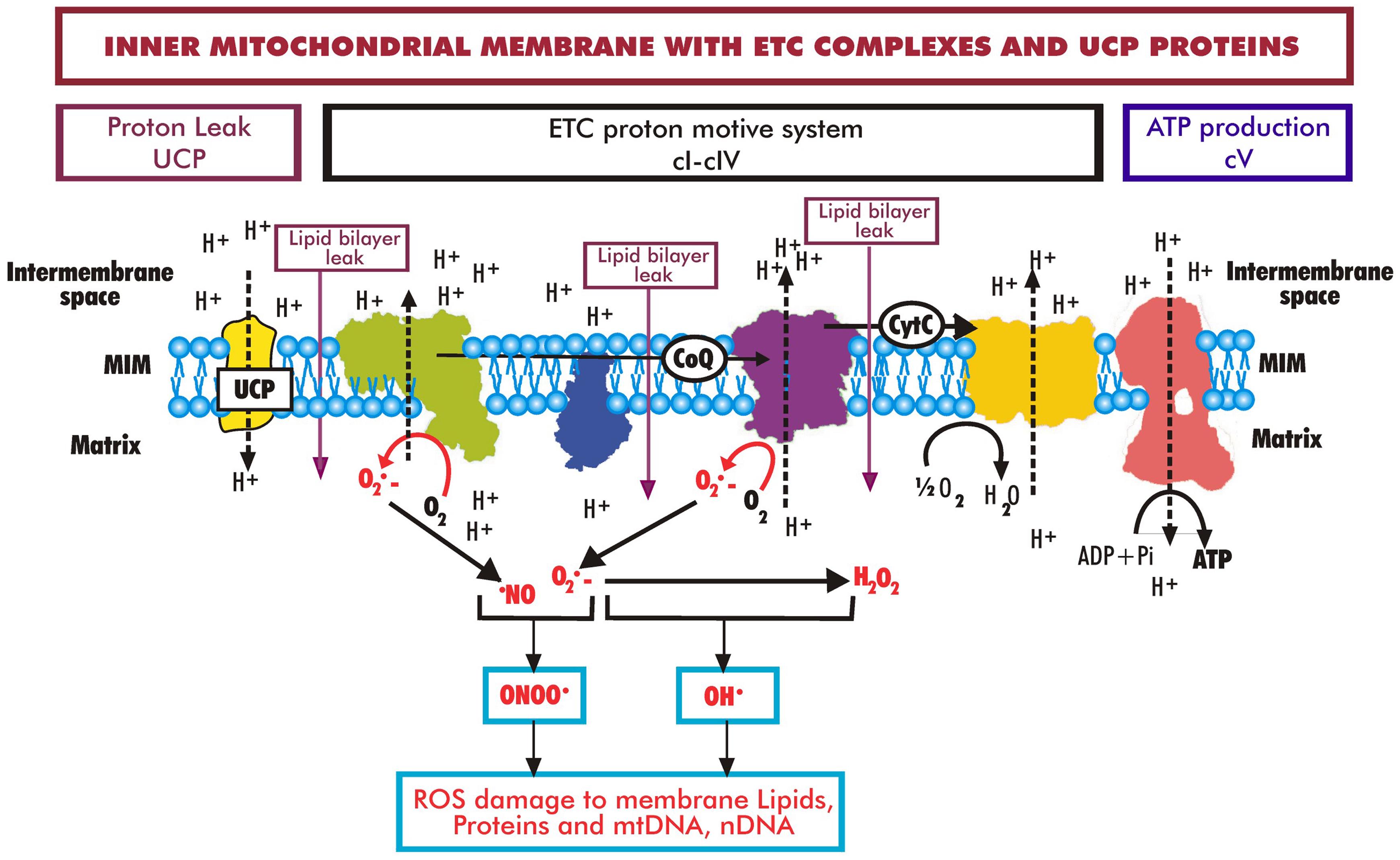
ROS are generated by mitochondrial complexes I and III (cI and cIII) during the transfer of electrons in the electron transport chain (ETC) that drives protons to the intermembrane space and establishes the transmembrane proton and electrochemical potential (ΔΨm ) across the MIM. Protons then flow back across the MIM through complex V (cV) to the mitochondrial matrix, forming ATP in the process. Some protons also flow back across the MIM due to uncoupling proteins (UCP), and some leak across the MIM lipid bilayer into the matrix (purple arrows). During aging, there is an increase in ROS generated by the ETC, resulting in increased oxidative damage to lipids, proteins, and DNA (modified from Grimm and Eckert and Nicolson et al.56,57).
It is now thought that a common characteristic of cellular membranes is the existence of distinct membrane regions with unique lipid and protein compositions, termed membrane domains.38,39,41,49 In these domains, the spatial organization, movements (such as rotational and lateral mobility), and lifetimes of molecular components are unlike the bulk or average properties of the membranes.38,39,49,50 These distinct physical characteristics include the inner and outer GPL leaflets that form the lipid bilayer. The FMM concept allows for the presence of membrane domains with unique compositions and mobility.39,49,50
We now know that cellular membranes are much more complex than implied by early membrane models. For example, considering only membrane lipids, they include various GPL, sphingolipids, sterols, and other lipids.37–39,49,50 Due to their cooperative properties and their propensity to match hydrophobic regions, various combinations of these lipids organize nonrandomly into lipid bilayers at physiological temperatures. Due to lateral segregation, they can also form nanometer-to-micrometer-sized domains with differing compositions.37,49,50 Lipid domains have unique compositions and properties different from the bulk membrane lipids. Some of these lipid domains can occur as islands encircled by disordered fluid-phase lipids.37–39,49,50
Age and various pathologies can change the biochemical characteristics and structural organization of cellular membranes. For example, as cells age, the fluidity of lipids at protein-lipid boundaries can change, as can the compositions of membrane lipid components.51 Other age- and disease-related changes in specific cellular membranes will be discussed in subsequent sections. Since membrane lipids are essential to the structure and function of biological membranes, when damage to membrane lipids occurs due to aging, trauma, or disease, the damaged lipids must be repaired or replaced to regain function. This is where membrane lipid replacement (MLR)—the use of protected oral membrane lipids to replace damaged membrane lipids in tissues and cells—can be used to restore membrane function.40,41 This will be discussed in more detail in subsequent sections.
Mitochondrial inner membranes
As essential organelles, mitochondria supply cells with important bioenergetic molecules as well as molecules involved in metabolic and signaling biochemistry. In addition, they are essential in providing innate immunity, programmed cell death, calcium and iron homeostasis, and other cell functions.12,17,18,23,52,53 All mitochondria have a double-membrane system similar to bacteria, their proposed ancestral origin.22,54,55 Here, we will focus on the inner membranes of mitochondria.
The most commonly known feature of the inner mitochondrial membrane (MIM) is the production of essential high-energy molecules, especially ATP, from the breakdown of lipid and carbohydrate substrates plus oxygen, which provide electrons to the components of the electron transport chain (ETC). This process is accomplished by utilizing a succession of redox reactions of five membrane enzyme complexes and cofactors like CoQ10, localized in the MIM (Fig. 2).56,57 The end products of this process are the reduction of molecular oxygen to water and the enzymatic synthesis of ATP.58–62
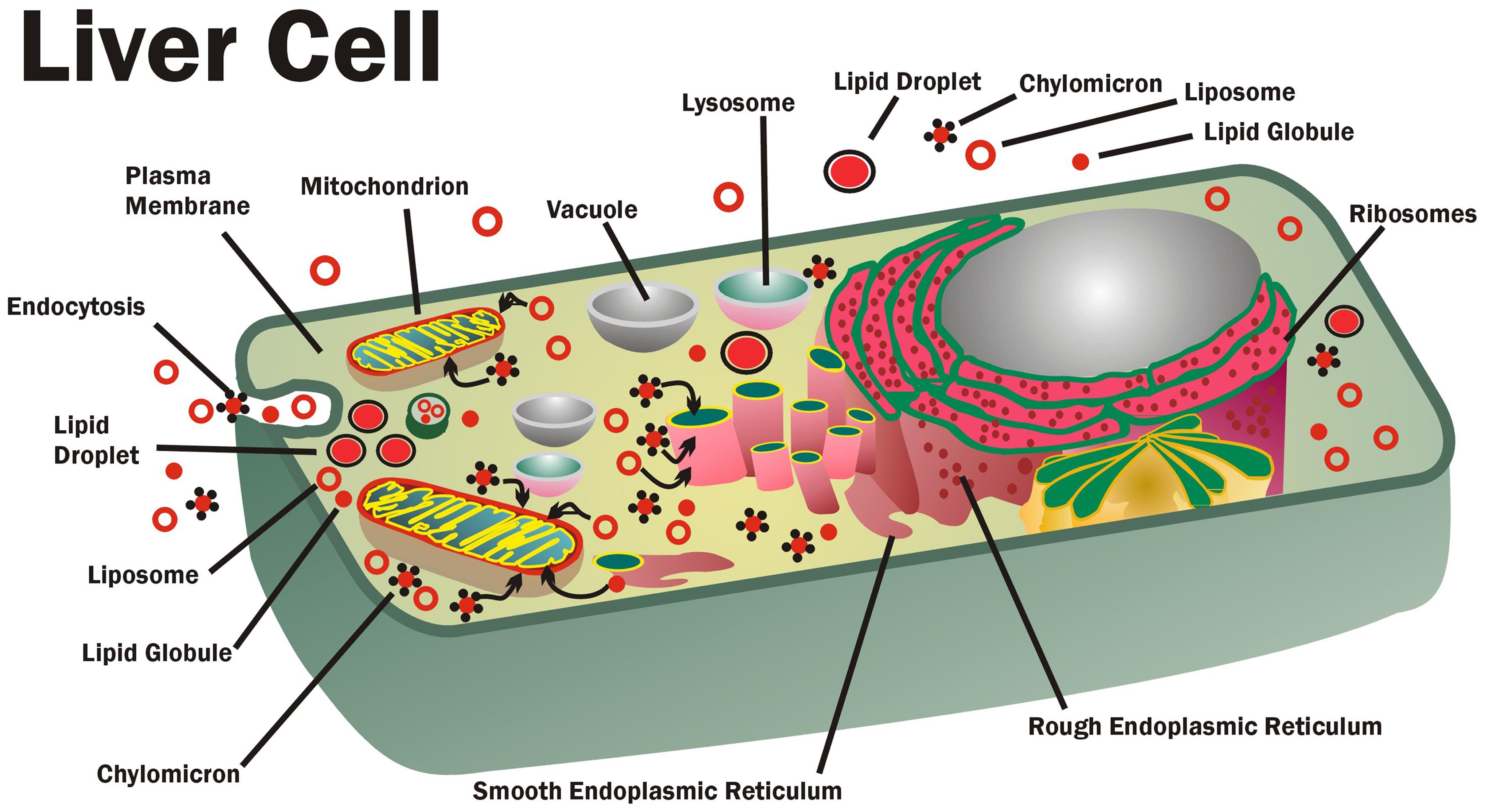
Membrane lipid replacement (MLR) glycerophospholipids (GPL) are transported into cells and into various intracellular membranes and lipid structures, such as lipoproteins, liposomes, chylomicrons, globules, lipid droplets, and other intracellular membranes. These structures can absorb damaged lipids, toxins, and toxic hydrophobic chemicals by partitioning and then remove and transport them from cells, where they are partitioned into other circulating lipid structures (modified from Nicolson).123
In addition to the ETC complexes in the MIM that contain components possessing iron-sulfur clusters, copper centers, and cytochromes necessary for the transfer of electrons, the MIM also utilizes flavins and CoQ10 in the electron transfer process. This process occurs as a sequence of oxidation-reduction reactions that generate an electrochemical gradient (ΔΨm) across the MIM.12,60,61 The MIM electrochemical gradient (ΔΨm of approximately −150 mV across the MIM under standard respiration conditions), or, if protons are considered, the proton motive force Δp, is then utilized by complex V to drive ATP generation from phosphate and ADP substrates.61–63 If the MIM ΔΨm (or proton electrochemical gradient (Δp)) cannot be maintained at −150 mV, complex V cannot flow protons across the MIM to synthesize ATP. When the MIM is compromised, for example, by free radical ROS/RNS damage to MIM lipids, increased numbers of protons can leak across the MIM. This most likely occurs at interface regions between modified lipids and other lipids, or between lipids bound to ETC complexes and bulk lipids, or through modified activities of MIM uncoupling proteins (see the diagram showing leak, proton flow, purple arrows, Fig. 2).61–64 The result is a decrease in Δp (and accordingly ΔΨm), which diminishes the ability of the MIM to synthesize ATP. Thus, it is absolutely necessary to maintain the integrity of the MIM in order to maintain Δp and ΔΨm so that cellular levels of ATP can be sustained.58,61–65 Moreover, some proton leak occurs naturally, and this may be necessary for thermogenesis, maintaining carbon flux, and modulating nutrient responses.66
ROS/RNS (ROS, composed primarily of hydroxyl radicals, superoxide anions, and hydrogen peroxide, plus RNS species like peroxynitrite) are formed at the inner side of the MIM during respiration as a byproduct of the flow of electrons through the ETC and synthesis of ATP (Fig. 2). Some production of ROS/RNS is necessary to maintain cellular signaling and other cellular processes like growth, proliferation, and apoptosis, but this process must be controlled.30 To control the proton gradient (Δp) and maintain redox balance, uncoupling proteins are present in the MIM to mediate the regulated discharge of the proton gradient so that the concentrations of ROS/RNS can be regulated (Fig. 2).63,66,67 Since oxidative phosphorylation is not completely coupled with ATP production, even in the presence of uncoupling proteins, excess ROS/RNS can be present above levels that are normally neutralized by endogenous cellular antioxidants. If the excess production of ROS/RNS is not carefully controlled, the result is damage to sensitive cellular lipids, proteins, and DNA.25,63,66–68 The excess ROS/RNS can efficiently cross the MIM and outer mitochondrial membrane and react with cellular components.25,63,66–69 If the damaged lipids, proteins, and DNA from this process are not repaired or replaced, the result is impaired function and loss of various cellular activities.56,59,62,69
Excess ROS/RNS produced by mitochondria and other mechanisms can directly damage various cellular membranes. In addition, ROS/RNS can have indirect effects on membrane functions by modification of non-membrane proteins.70,71 Much of the damage to membranes occurs when ROS/RNS create lipid dienes, hydroperoxides, and other lipid modifications. Of particular importance is the formation of lipid peroxides in polyunsaturated fatty acids (FAs) of GPL that are unstable and result in products like malondialdehyde and 4-hydroxy-2-nonenal.25,71 Protein oxidation by ROS/RNS can also result in protein carbonyls and other oxidation products that can indirectly affect enzyme and membrane protein functions.25,70 Gutteridgehas outlined the types of ROS/RNS membrane and cellular modifications that have been implicated in or are components of aging and disease pathology, including diseases involving excess generation of superoxide, hydrogen peroxide,28 and hypochlorous acid; drug-induced ROS; electron transfer by transition metals; excessive substrate oxidation or changes in oxygen concentration; failure of host protective defenses, and excess free radicals affecting other structural changes or perturbations in cells.
The deterioration of mitochondrial and other cellular functions is known to occur with advancing age. This is largely thought to be due to the over-production of excess ROS/RNS and their reaction with MIM lipids, cellular proteins and membranes, mitochondrial (mtDNA) and nuclear DNA, and other mechanisms.12,22,72 If not limited, this deterioration can result in mitochondrial destructive processes or mitophagy.12,19,72–76 Such observations have stimulated proposals that the aging of animals and their lifespans are regulated by their mitochondria.26,27,77 The main source of excess cellular ROS/RNS is definitely the mitochondria.25,73,76 Even if low levels of ROS/RNS production are necessary to maintain normal cellular physiology, especially intracellular signaling, excess production above levels neutralized by endogenous antioxidants can result in irreversible damage to critical cellular structures.25,29,52,78 Although mitochondrial decline is definitely associated with aging,21,72 there are reasons to doubt the hypothesis that aging is solely controlled by mitochondrial ROS/RNS.12,21,31
Membrane GPLs that form the lipid matrix of the MIM and other intracellular and plasma membranes are particularly sensitive to ROS/RNS injury. Among the most ROS/RNS-sensitive, functionally important phospholipids in the MIM is cardiolipin (CL).79 Unique to mitochondria, CL constitutes up to 20% of MIM phospholipids. CL is essential for mitochondrial membrane fluidity, osmotic stability, ETC function, and maintenance of ΔΨm.80,81 Therefore, ROS/RNS damage to CL in the MIM can result in ETC dysfunction, the collapse of ΔΨm, and eventually mitophagy.82 Thus, CL is considered a critical target for mitochondrial dysfunction.83
In addition to CL, ROS/RNS oxidative damage can also distort and disrupt other mitochondrial membrane lipids, such as certain unsaturated GPLs. The unsaturated FAs in GPLs are very susceptible to damage by ROS/RNS.25,84
Mitochondrial oxidative events can interfere with membrane organization, especially lipid-lipid and lipid-protein interactions, and they can modify MIM lipid fluidity, which is necessary for ETC function.84,85 Eventually, ROS/RNS damage to the MIM also increases proton leak across the MIM, resulting in reduced Δp (and ΔΨm ) and loss of ATP production. Indeed, there is a direct association between ROS/RNS damage to unsaturated mitochondrial lipids and loss of mitochondrial function.86 Thus, as membrane lipids, especially those found in mitochondria, are injured by ROS/RNS, they must be continuously replaced or repaired to maintain mitochondrial and cellular functions at normal levels.
Membrane ion channels
Membrane ion channels are constructed from pore-forming proteins that span membrane lipid bilayers and allow ions to move across those membranes at high rates. Ion channels can dissipate the energy of membrane electrochemical gradients, such as in the MIM, and in the process, convert that energy into chemical, physical, or electrical signals. Ion channels are implicated in several biological processes involving intracellular and plasma membranes, and they are importantly entangled in the aging process and age-related morbidities.7,13
Ion channels have been found to be important in age-associated neurological and vascular diseases, such as Alzheimer’s disease, various cardiovascular disorders, and disease-associated pain and inflammation. Ion channels have also been used as targets for therapeutic treatments for various conditions.7,13,87 In particular, changes in ion channel function have been implicated in the aging of the brain and in furthering the deterioration of cognitive function, which is a facet of normal aging.88 The main mechanisms of damage during brain aging are inflammation and oxidative stress, both of which can result in cell degeneration, cognitive decline, and death.72 In the brain, ROS/RNS species and the subsequent oxidation of different proteins (especially in membranes and their ion channels) have been proposed to participate in the aging process.89 In particular, calcium and potassium channels, such as calcium-activated potassium channels (Slo1 or K(Ca) channels), are likely involved in the mechanisms that underlie cognitive decline.88,90 Although there are many types of calcium/potassium channels or K(Ca) channels, most of them are modulated by lipids.91,92 As will be discussed below, it has been observed in several clinical studies that supplementation with exogenous lipids and replacement of critical GPLs improved cognition in aged individuals.
Another example of how ROS/RNS damage to ion channels can affect cells is their effect on L-type voltage-sensitive calcium channels. The opening of L-type voltage-sensitive calcium channels can be stimulated by ROS, resulting in excessive increases in intracellular calcium concentrations. This has been observed in neurodegeneration, stroke, and other conditions where excess generation of ROS/RNS occurs along with increased Ca2+ concentrations.93,94 This is of particular importance since L-type Ca2+ channels are a key player in Ca2+ homeostasis, which is also known to play a role during aging.95 Other ion channels and ion transporters are also critical in the maintenance of intracellular calcium homeostasis; however, a discussion of these topics is beyond the scope of this review (see ref. 96 for additional information on this topic).
Apoptosis is another cellular event where ROS/RNS damage to mitochondrial permeability transition pores (mPTP) can cause its initiation. This is thought to be caused by oxidation of tightly protein-bound lipids that can accelerate the calcium release process and result in a chain of events described as calcium-dependent apoptosis.97 Thus, ROS/RNS-induced increases in the permeabilities of certain membrane channels can affect Ca2+ homeostasis. It has been observed during in vitro studies that certain cell disturbances, such as increased intracellular Ca2+ concentrations due to exposure to oxidizing agents, could be minimized when cells were incubated with replacement phospholipids.98
In the mitochondria, ion channels have been determined to be indirectly or directly associated with aging and age-related conditions.13,99 For example, MIM ion channels are crucial in regulating mitochondrial function, and their dysfunction has been connected to aging and age-linked diseases. Impaired oxidative phosphorylation activity, increased ROS/RNS release, and decreased ATP production are all related to aging.12 Ion channels in mitochondria have been proposed as the gatekeepers of life and death, and most cellular energy production depends on fully functional mitochondria, which in turn depends on regulated ion channels.13,100
As discussed above, the activity of a critical mitochondrial membrane channel that is important in aging and age-associated degenerative disorders, the mPTP,99,101 is enhanced in aging. It has also been established to be important in many age-associated degenerative conditions.102,103 An important role of the mPTP is the passage of ROS/RNS from mitochondria into cells, which is mainly dependent on the mPTP. When the mPTP is stimulated by calcium ions, oxidative stress, or depolarization of the MIM, the result is that protons move into the mitochondrial matrix, leading to the collapse of Δp and ΔΨm and the loss of ATP production. In the process, superoxide, hydrogen peroxide, calcium, and various other ions flood through the mPTP and exit the mitochondria into the cytoplasm, where subsequent damage occurs.102,103 Eventually, prolonged activation of the mPTP can result in cell demise by necrosis or apoptosis.101
Therefore, the mechanisms of ion transport in cell membranes and mitochondria are of major regulatory importance in pathways that can determine the rates of cell or organism aging and death, and some membrane channels have been proposed to function at the intersection of one or more of the control points of aging.13,100 Interestingly, most mitochondrial ion channels are modulated by lipids, suggesting that membrane lipids might be a suitable therapeutic agent.104–106
MLR
ROS/RNS-damaged membrane phospholipids cannot be easily repaired, and the exchange and replacement of oxidized/damaged membrane phospholipids with undamaged, functional membrane phospholipids are crucial for mitochondrial function as well as for multiple cellular and tissue functions that are essential for maintaining general health.107–110
When dietary lipids are ingested, they are almost exclusively absorbed in the small intestine, where enzymatic and non-enzymatic degradation can occur. The degradation products are then absorbed by epithelial cells.111,112 However, when present in excess of degradative enzymes in the small intestine, GPL and other lipids can also be transported through the epithelial lining within small globules, liposomes, and other lipid structures. The GPL subsequently find their way into the portal blood circulation to the liver in relatively undegraded forms.113 This type of absorption is very efficient, and more than 90% of absorbed GPL make their way from the gastrointestinal system into the blood circulation within six hours.112–115
Dietary lipids, like GPL with unsaturated FAs, are very sensitive to oxidation and degradation throughout the various steps in their preparation, storage, ingestion, and absorption.116 To avoid damage and degradation, dietary GPL have been supplemented with protective plant inulins (fructooligosaccharides). When added to GPL formulations, the inulin molecules insert themselves among the head groups of the GPL and the hydrophobic lipid tail bilayer, shielding these lipids with their double bonds from the effects of high or low temperatures, degradative enzymes, bile salts, acidity, and various oxidative events.117,118 Such complexed, protected GPL form the basis of oral MLR, and these protected GPL are essential for the removal and replacement of damaged membrane lipids.40,110,119,120
In the liver, some of the GPL are absorbed by liver cells as individual molecules transferred mainly from lipoproteins to the plasma membrane. Alternatively, when they are present in excess, they are mostly moved by bulk transfer or endocytosis of liposomes, lipid globules, and other forms into endosomes.120–122 Once inside liver cells, the inulin-protected GPL can partition into various intracellular membranes and other hydrophobic structures, including liposomes, lipid globules, droplets, chylomicrons, and other phospholipid-containing structures inside various cells (Fig. 1).123 The transfer and movement of most phospholipids inside cells occur by a process of contact-mediated partitioning.124,125 When they reach their intracellular destination sites, the membrane lipids can be enzymatically modified, and they will eventually reflect the lipid compositions usually found at these locations.126 In reverse, oxidized or partially degraded phospholipids can be exchanged and displaced from membranes and other structures. The exchanged lipids can partition back into lipid globules, liposomes, chylomicrons, and other intracellular lipid structures. Eventually, the damaged lipids can be partitioned or transferred into membrane pre-exosomes for secretion or sequestered into other lipid structures destined for secretion from cells by exocytosis.110,119–122
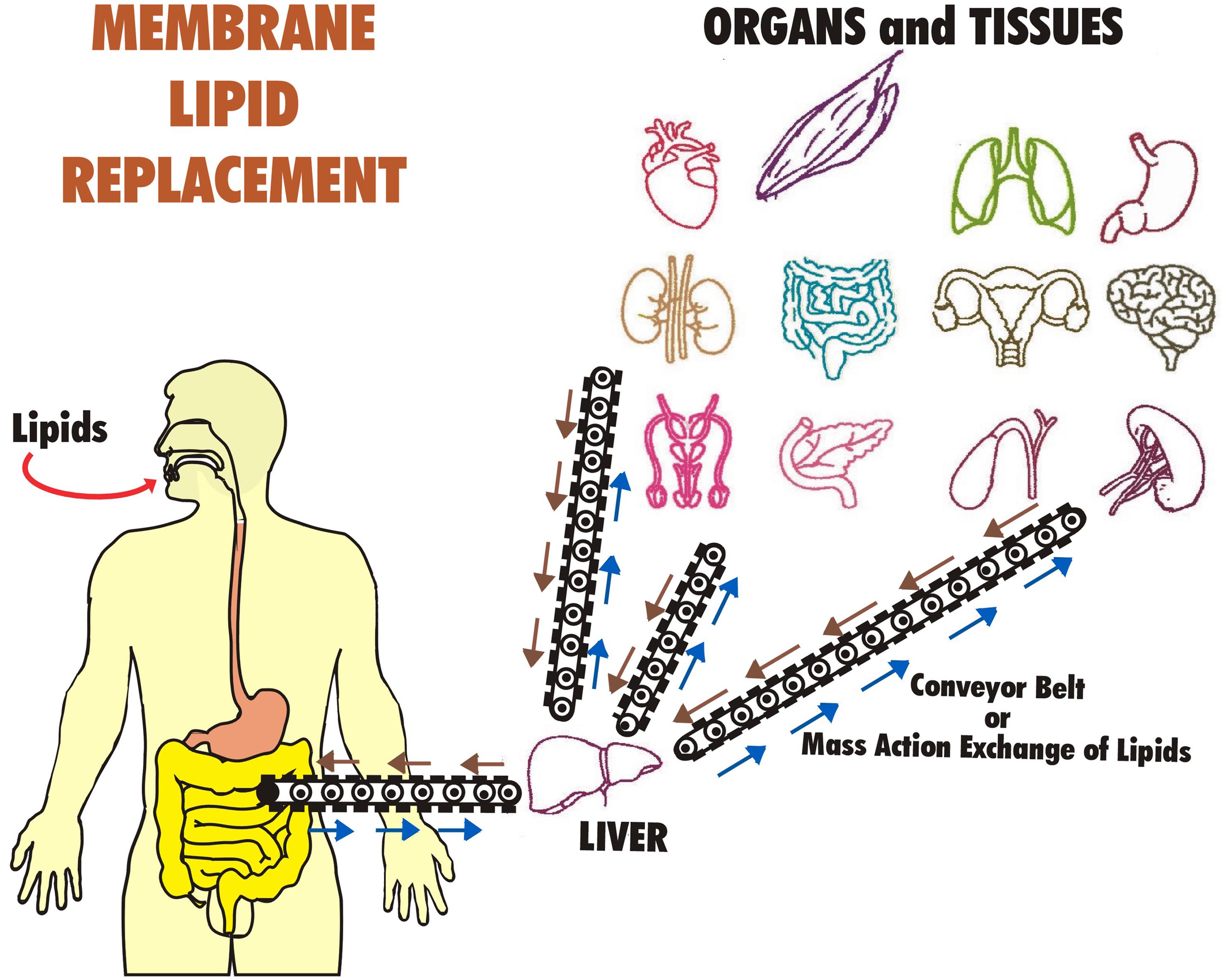
The exchanged, damaged lipids that are secreted from tissues and organ cells into the blood circulation and lymph are eventually returned to the gastrointestinal system for bulk export in the stool.110 This entire process operates on a ‘mass action’ or ‘bulk flow’ system that acts like a conveyor belt, delivering undamaged replacement lipids to cells and tissues,122 while simultaneously acting as a removal and disposal system for damaged, oxidized, and degraded lipids (Fig. 3).110,119–123
There, the GPL are partitioned and exchanged into cells, intracellular membranes, and other intracellular lipid structures (lipoproteins, liposomes, chylomicrons, globules, lipid droplets, etc.). When GPL are in excess, the reverse process also occurs, and the excess replaced GPL (along with other hydrophobic molecules) are transported back to the gastrointestinal system for export in stool.
Because of the fluidity of mammalian cell membranes and the presence of fusogenic membrane domains, transported lipids can fuse with various cellular membranes and eventually with the intracellular membranes of all organelles.124,125 For example, we have observed in living cells that added GPL nanomicelles are first incorporated into the plasma membranes and then partitioned into intracellular membranes, including mitochondrial membranes.98
Maintaining an appropriate diet of undamaged phospholipids is the usual method for supplying and exchanging membrane lipids and driving the removal of damaged lipids from cells and tissues. However, the exchange and eventual removal of damaged membrane lipids using diet alone is generally impractical and, for the most part, rarely achievable.110,120,123 Also, most dietary sources of membrane lipids are usually not inulin-protected from oxidation and degradation, so damaged lipids could be introduced into cells and tissues from dietary sources. Thus, the dietary supplementation of protected MLR lipids, along with antioxidants, provides a convenient method to deliver therapeutic doses of important membrane lipids in undegraded, unoxidized forms to maintain cellular and tissue membrane function.109,110,119,120,123
An important point is that dietary MLR supplements are extremely safe. There have been no indications of toxicity in preclinical or clinical studies using MLR phospholipids. Nor has any maximum dose level been achieved in multiple animal and human studies (reviewed in ref. 110). The U.S. Federal Drug Administration has determined that oral MLR supplements made up of membrane GPL are “generally regarded as safe”.127 A variety of MLR supplements are available commercially; a few of these are listed in Table 1.107,109,110,119,128–131
Examples of MLR dietary glycerophospholipid supplements*
| Source | Product name | GPL composition** (%) | Reference |
|---|---|---|---|
| Soy | Essentiale® | 72 PC, others: PS, PE, PI | Gundermann107 |
| Soy, Sunflower | NTFactor® Lipids | 31.6 PC, 25 PI, 19 PE, 13.9 PA, 5.9 DGDG | Nicolson119 |
| 2.4 PG, 1 LPC, 0.5 PS, 0.7 LPE, 0.3 MGDG FA: 58 LA(n-6), 16 PlA, 9.7 OA, 5.9 LA(n-3) 3.9 SA | Nicolson & Ash110 | ||
| Soy | Lipoid S45® | 45–50 PC, 10–18 PS, 4 LPC FA: 58–65 LA, 12–17 PlA, 8–12 OA | Eros et al.128 |
| Soy | PC55® | 45–50 PC, 10–18 PE, 4 LPC FA: 58–65 LA, 8–12 OA, 12–17 PA | Ladd et al.129 |
| Soy | Lecithin | 40 PC, 20.8 PE, 3 PS, 2 PA | Wilson et al.130 |
| Marine oil | Vitalipin® | 40 PL, 15 EPA, 9 DHA | Küllenberg, et al.109 |
| Bovine milk | Lacprodan® | 27 PC, 22 PE, 27 SPM, 12 PS, 8 PL 0.1 ArA | Ohlsson, et al.131 |
In the following sections, some of the potential uses of MLR in preclinical and clinical studies are reviewed. The most obvious limitation in these studies is that they are, in general, preliminary or pilot studies, not comprehensive, multiple, multi-centered, randomized, controlled trials. This is mainly due to the severe limitations in resources to support research and clinical studies on natural supplements, and the fact that this is a relatively new area of investigation.
MLR preclinical studies
The preclinical uses of various MLR phospholipids have been reviewed previously.109,110 We will only summarize some of the literature on this subject here. Laboratory animal disease models have been used to study the benefits of MLR supplements on various health conditions. For example, the benefits of various membrane phospholipids have been studied with respect to inflammatory processes, such as in rodent models for leukocyte-dependent arthritis.128,132 Using a murine model for chronic rheumatoid arthritis, Eros et al.128 found that an oral mixture of soy phospholipids limited inflammatory processes in joints. Other uses of membrane GPL to study their effects on inflammation-associated conditions are reviewed elsewhere.109,110
Different animal tumor models have been utilized to study the benefits of GPL in the suppression of tumor formation in laboratory animals, tumor cell proliferation in culture, and growth in vivo.109,110 As an example, MLR supplements have been used to decrease the incidence of hepatocarcinoma formation in rodents and other tumors.133,134 Elsewhere, bovine milk phospholipids were used to prevent gastric tumor formation in older rats,135 and an oral mixture of sphingomyelin and other phospholipids was found to inhibit colon tumor incidence in aging mice.136
The effects of MLR lipids have also been studied in animal models developed to analyze metastasis and secondary tumor formation. For example, the effects of hydrogenated phosphatidylcholine (PC) plus cholesterol on an orthotopic pancreatic metastatic tumor model in mice were investigated by Graeser et al.137 Although they found that the growth of primary implanted tumor cells was not significantly affected, distant metastasis formation was inhibited by the MLR lipid formulation. The active factor was identified as a PC degradation product, lysophosphatidylcholine. The LysoPC degradation product was rapidly taken up by the pancreatic tumor cells, and there was a significant increase in hydrogenated FAs in the pancreatic tumor cell membranes, resulting in a loss of adhesion properties, especially to platelets and endothelial cells. In a murine B16 melanoma model to study experimental metastatic pulmonary tumor formation, pretreatment of B16 cells with hydrogenated lysophosphatidylcholine decreased the number of experimental lung metastases by half.138 As mentioned above, one step that could be affected by MLR in the metastatic process is the adhesion of circulating malignant cells to endothelial cells, and in some cases, to platelets. This can result in tumor cell emboli that lodge in the microcirculation. The embedding of tumor cells and tumor cell emboli in the microcirculation is known to be an important step in the formation of distant metastases.139,140
Aging-associated sensory deficits are also modified by MLR phospholipids. An interesting preclinical study on MLR and aging involved aged rats and age-associated loss of hearing.141 Age-related hearing loss is well known in these animals and can be monitored by auditory brainstem responses (ABR). In rats, hearing loss becomes significant after 20 months of age. In this study, MLR GPL lipids were given to older (18–20-month-old) rats in their chow for six months, and ABR responses were assessed and compared to control untreated rats. Mitochondrial ETC function, a parameter known to decline with age, was determined by measuring the transmembrane electrical/chemical potential (ΔΨm) of blood leukocytes using the redox dye Rhodamine 123 and monitoring fluorescence at 530 nm by cell cytofluorography. This dye partitions into the MIM and reports ΔΨm in live cells. Mitochondrial mtDNA deletions were also monitored during the study by amplifying specific mtDNA sequences (ND1-16srRNA and other mt-rRNA sequences) and tracking deletions in specific mtDNA sequences in each group of animals. After four to six months on the MLR dietary supplement, Seidman et al.141 found significant preservation of the hearing threshold at several frequencies in the experimental group of rats, whereas in the control group, the hearing loss measured by ABR responses was significant at various frequencies. The MLR supplement also prevented loss of mitochondrial function measured by a reduction of ΔΨm in blood leukocyte mitochondria, as shown by MIM fluorescence of Rhodamine 123. The MLR supplement also lowered the number of mtDNA deletions found in the aged rats. Therefore, the MLR test group of aged animals receiving the MLR GPL were protected against age-related loss of ABR, MIM ΔΨm, and increases in mtDNA deletions compared to the control group.141
Some clinical uses of MLR in aged subjects
Several case series and clinical trials have studied the effects of MLR supplements on various age-related clinical conditions (Table 2).57,98,107,109,110,120,123,142–159 We will only discuss a few clinical studies in the context of aging. These examples should not be considered comprehensive, but they are typical of the types of clinical MLR studies that have been used to examine the effects of MLR on aging and age-associated chronic illnesses.
Some uses of oral MLR supplements and suggested daily dosesa
| MLR Use | Subjects/uses | Age group | MLR supplement | MLR doseb (g/day) | Reference |
|---|---|---|---|---|---|
| Fatigue | Aging, fatigue | Senior | NTF/L | 4 | Agadjanyan et al.142 |
| Fatigue | CFS/ME | 18–72 | NTF/L | 4 | Nicolson & Ellithorpe143 |
| Fatigue | CFS/ME | Adult | ATP Fuel | 4 | Nicolson et al.144 |
| Inflam/fatigue | Chronic fatigue | 18–75 | ATP360 | 0.4 | Hamilton & Jensen145 |
| Fatigue | Fibromyalgia | Adult | NTF/L | 4 | Nicolson et al.146 |
| Fatigue/pain | Menopause | Senior | NTF/L | 3 | Hirose et al.147 |
| Weight loss | Obesity, fatigue | Adult | NTF | 3–4 | Ellithrope et al.148 |
| Brain health | Neurodegen. dis. | Adult | NTF/L | 4 | Nicolson et al.149 |
| CD health | CD risk/CD dis. | Senior | NTF/L | 4 | Ellithorpe et al.150 |
| Metabolic health | MetSyn/diabetes | Adult | NTF/L | 2–4 | Nicolson151 |
| Metabolic health | Diabetes | Adult | ATP Fuel | 3–4 | Nicolson et al.144 |
| Neurobehavior | Autism Sp. dis. | Child | NTF/L | 1–3 | Nicolson et al.149 |
| Fatty Liver | Fatty liver dis. | Adult | EPL | 1–1.8 | Gundermann et al.152,153 |
| Infection | Lyme dis. | Adult | ATP Fuel | 4 | Nicolson et al.144,154 |
| Infection | Mycoplasma | Adult | NTF/L | 3–4 | Nicolson155 |
| Fertility | Fertility test | Adult | NTF/L | NAc | Ferreira et al.98 |
| Fatigue | Br cancer | Senior | NTF/L | 4 | Nicolson156 |
| Anemia | Anemia | Adult | NTF/L | 4 | Ellithorpe et al.157 |
| Injury | wounds | Adult | NTF/L | 4–6 | Nicolson & Breeding158 |
| Autoimmune | Rheumatoid arthritis | Adult | ATP Fuel | 4 | Nicolson et al.144 |
| Chemical detox | GW Illnesses | Adult | NTF/L | 6 | Nicolson & Breeding159 |
| General health | Aging | Senior | NTF/L | 3–4 | Nicolson et al.57,110,120 |
Chronic fatigue and fatiguing illnesses
One of the most advantageous clinical uses of GPL supplements has been to remedy fatigue. Fatigue is the most common symptom conveyed to practitioners by aged and chronically ill patients.160 Moreover, independent of age, fatigue is a common and routine complaint of patients under general medical care, and it is especially prevalent in patients over the age of 50. Fatigue has been closely associated with advanced age in several clinical studies.161
Fatigue is also one of the most commonly found co-symptoms in most, if not all, chronic morbidities.160,161 Although it is considered a complex sensation that is not fully understood, fatigue is generally recognized as a reduction in overall energy, including both psychological and physical tiredness. Most patients report physical exhaustion, diminished physical endurance, and a reduced ability to complete routine tasks without excessive physical effort. Fatigue is particularly apparent in aged individuals and those with chronic disease conditions, likely due to several factors, especially the well-documented reduction of mitochondrial function with age.161–163 Moderate and severe fatigue have been correlated with a loss of mitochondrial function and reduced ATP synthesis by the mitochondrial ETC. However, mild fatigue can be impacted by other causes, such as depression or psychiatric conditions.163,164 Therefore, most studies related to aging have focused on moderate to severe fatigue. Fatigue that is continuously present for more than six months is typically termed chronic fatigue, or, in some cases with additional symptoms, chronic fatigue syndrome or myalgic encephalomyelitis (hereinafter referred to as CFS/ME).163
MLR GPL supplements have been successfully used to reduce fatigue in adults with moderate to severe fatigue, as well as in adults with chronic fatigue (fatigue lasting at least six months) (reviewed in Ref. 110,118,120). One example of such a study is a clinical cross-over trial using MLR GPL in elderly subjects with moderate to severe chronic fatigue. The results of this study showed a statistically significant correlation between reductions in fatigue and improvements in mitochondrial function, as determined by MIM transmembrane potential in peripheral blood leukocytes.142 After eight weeks of an oral GPL supplement (4 g/day), mitochondrial function (measured as MIM ΔΨm ) in patients with moderate to severe fatigue improved significantly, and fatigue was reduced significantly. After eight to twelve weeks on the oral GPL supplement, mitochondrial function was enhanced by 35% (p < 0.001), and fatigue decreased significantly (36%, p < 0.001). Interestingly, the men in the study performed slightly better than the women. The improvements found after 12 weeks on GPL showed that mitochondrial function improved to levels similar to those found in 30-year-old healthy adults. After the 12-week GPL supplementation, all participants were switched to a placebo formulation (without their knowledge) for an additional 12 weeks, and their fatigue and mitochondrial function levels were recorded. After the 12-week placebo period, fatigue and mitochondrial function were intermediate between the initial values and those found at 12 weeks on the GPL supplement. Therefore, in aged individuals with moderate to severe chronic fatigue, there were highly significant improvements in fatigue scores while on the MLR supplement, and mitochondrial function increased to levels similar to those found in much younger adults, but only if the study participants maintained their daily use of the GPL supplement.142
Similar to aging adults with chronic fatigue, the MLR GPL supplement was found to reduce fatigue in patients diagnosed with CFS/ME, fibromyalgia, Gulf War illnesses, chronic infections (such as chronic Lyme disease and Mycoplasma infections), menopause, and other conditions (Table 2). MLR also appears to be useful in reducing cancer-related fatigue.119,156
In some of the MLR clinical studies, results could be compared between adults (<50 years old) and more senior participants (>60 years old). In multiple studies, both younger and older subjects gained significant benefits from the use of GPL supplements. Often, there were greater improvements in fatigue scores in the older subjects compared to those less than 50 years of age; however, statistical comparisons of the differences between these age groups usually failed to reach significance. This strongly implies that both older and younger subjects can benefit from MLR supplementation to reduce chronic fatigue.
MLR has also been used in combination with mitochondrial supplements containing additional ETC cofactors and other ingredients to boost mitochondrial function and diminish fatigue and other symptoms.142,144,146,149,150,154,157 One of the supplements listed in Table 2 employed a combination MLR formulation containing NADH, CoQ10, and other ingredients that promote mitochondrial function.144 Different MLR supplements have been employed to reduce fatigue in intractable chronic fatiguing illnesses and also in chronic infections like chronic Lyme disease and other conditions.144,154 There are a number of possible nutrient and supplement combinations that can be used to increase mitochondrial function.144,145,162,164 We have only discussed a few here.
Pain and associated symptoms
Along with fatigue, pain is a common complaint in patients with chronic illnesses and aged individuals.165,166 In higher animals, neurotropic pain is transmitted via three primary types of afferent nerve fibers: (1) heavily myelinated mechanical-afferent nerves responsible for non-noxious tactile sensations; (2) myelinated, small-diameter nerve fibers that transmit signals of sharp pain; and (3) non-myelinated, small-diameter nerve fibers that transmit dull, aching pain.167 In addition, pain signals can also be amplified in the brain.168
Recently, MLR supplements have been utilized clinically to reduce widespread fibromyalgia pain and other forms of pain.146,158 The reason MLR supplements have only been used recently to reduce pain is that this appears to require much higher daily MLR phospholipid dosing than is necessary to alleviate other symptoms like fatigue.158,159 In recent clinical case studies and small clinical trials on pain reduction, the usual daily doses of MLR GPL were much higher (4–6 g/day) than those typically given for fatigue reduction (2–4 g/day).158,159 Only the higher doses (6 g/day) were truly effective in reducing widespread pain, as well as pain due to various causes, including chronic pain from gastrointestinal symptoms, trauma from gunshot wounds, and other sources of pain.158,159 In the case of widespread chronic pain, such as found in fibromyalgia, 4.8 g/day of oral GPL for eight days was found to significantly reduce pain (p < 0.001), fatigue (p < 0.001), and gastrointestinal symptoms (p < 0.001), while improving quality of life measurements (p < 0.001).146
For widespread pain and other symptoms often accompanying pain, more complex symptom assessments have been used to track patients’ signs and symptoms. For example, a six-month study of chemically exposed veterans assessed the severities of more than 100 signs and symptoms, self-reported approximately monthly by participants.159 In this open-label study, chemically exposed veterans (average age>50) taking 6 g/day oral MLR GPL recorded their symptom severities over time. By the end of the study, reports indicated gradual and significant reductions in pain, fatigue, and other symptoms during the study, employing redundancy to confirm symptom severities.159 This clinical study followed from a case series where the severity of pain was a significant clinical feature.158 In this case series, 50+-year-old veterans with severe pain initially took MLR GPL at lower doses (2–3 g/day), but the dose was later adjusted upward to 4 g and then 6 g/day to control pain. A notable subject was a 68-year-old female with previous gunshot wounds to her abdomen and lower back. For several decades, this patient experienced unrelenting chronic fatigue, pain, diarrhea, gastrointestinal, and other unresolved symptoms, even with strong pharmaceutical pain control. She discontinued narcotic pain treatment and was placed on MLR GPL at a dose of 5 g/day, along with myofascial trigger point therapy and spinal manipulation. Her pain, fatigue, and other symptoms improved over time, and she was able to regain control over her bowel movements. After three weeks of MLR, her pain, fatigue, and gastrointestinal symptoms improved markedly. However, when she later discontinued MLR supplementation, her symptoms slowly returned.158 This demonstrates that symptoms like chronic fatigue (discussed earlier) and pain require continued MLR treatment to be clinically effective.
Pain is a complex sensation, and one type, nociceptive pain, has been described in both acute and chronic forms as sharp or throbbing pain sensations in the muscles, joints, tendons, skin, and other tissues. Although this type of pain is usually short-lived, it can also become chronic and is thought to be part of the body’s general response to potentially harmful stimuli to protect against potential damage. Nociceptive pain has been subdivided into two classifications: (1) somatic nociceptive pain, which is usually localized in the dermis, and (2) visceral nociceptive pain, which is more diffuse, with poorly defined sensations in the body midline.169 Multiple stimuli can activate nociceptors to create pain. One of the groups of membrane ion channels involved in nociceptive pain transmission is recognized as transient receptor potential (TRP) channels.170 TRP channels are modulated by membrane GPL, such as phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), which acts as an agonist with desensitization properties. The addition of PI(4,5)P2 has been shown to inhibit heat- and capsaicin-activated transient receptor potential channels of the vanilloid subtype 1 (TRPV1), and the hydrolysis of PI(4,5)P2 by phospholipase C activation can reverse this inhibition, potentiating transient receptor potential channels of the vanilloid subtype 1 activity by pro-inflammatory agents.171 Although TRP channels are activated by PI(4,5)P2, they can quickly become unresponsive (desensitized) and lose their ability to be stimulated. Also, phospholipase C can hydrolyze PI(4,5)P2, yielding two classical second messengers (inositol 1,4,5-trisphosphate and diacylglycerol). Thus, small amounts of certain phospholipids, such as PI(4,5)P2 present in some MLR formulations, may be essential in producing the pain-reducing properties of this MLR supplement.40
Cognition and neurological symptoms
As adults age, neurological alterations occur, leading to memory loss and other changes, which have been correlated to differences in the lipid compositions of brain cells compared to other tissues. For example, the amounts of n-3 polyunsaturated FAs in brain GPL tend to decrease with age, which can have profound effects on membrane fluidity, membrane domain formation, and the function of membrane channels.172
In aging subjects, MLR phospholipids have been used to prevent memory loss. For instance, the effects of MLR phosphatidylserine (PS) have been studied in a clinical trial involving aging patients with Alzheimer’s disease.173 The participants in this study ingested 300 mg of PS daily for six months. At the end of the six-month period, the subjects showed significant improvements in cognition compared to controls without PS.173 In a separate study using elderly subjects with age-associated memory impairments, who ingested 300–600 mg/day of soybean PS for a much shorter (12-week) period, the results could not be duplicated, suggesting that dose and duration of treatment may be important.174
Short-term changes in mental clarity and focus after taking MLR phospholipid supplements have also been assessed. In one study, subjects consumed 0.6 g of MLR GPL in a liquid formulation. After three hours, fatigue and mental focus were assessed using a self-reported survey. Most of the participants reacted to the MLR GPL supplement within 1 h, and by 3 h, there were self-reported improvements in cognition, mental clarity, and focus, along with reductions in perceived fatigue.175 However, the results of such very short-term, subjective studies are difficult to interpret due to the inability of subjects to record consistent assessments. In most reported MLR studies, improvements in various symptoms did not occur that quickly—more time, usually days or weeks, was required to see consistent, significant results. There was, however, a suggestion that higher daily doses of MLR supplements could speed this process.158,159,176 This phenomenon could be similar to the ‘mass action’ or ‘bulk flow’ mechanism of MLR, where greater amounts of GPL may speed up the replacement and removal process.122
In these and other clinical studies, improvements in cognition, mood, focus, and other parameters were recorded, for example, as part of a self-reported survey developed by Piper et al.177 for monitoring cancer-related fatigue. Use of a more extensive survey form that tracked the severities of >100 signs and symptoms may yield more detailed information.159 Using more complex survey forms completed repeatedly over several weeks (or even several months) resulted in more significant and consistent improvements in cognition, mood, and other parameters in patients using MLR supplements.
Gastrointestinal symptoms
Two of the benefits of oral MLR phospholipids are that they appear to repair the gut epithelium and, in the process, reduce gastrointestinal symptoms.134,146,158,159 This has important implications for microbial translocation disorders and leaky gut syndromes, where pathogenic microbes can penetrate the damaged gut epithelial layer, enter the circulatory system, and elicit strong host responses.178
MLR GPL has been found to reduce abdominal symptom severities and repair gut epithelium, and this has occurred as a byproduct of the use of oral MLR to reduce other symptoms. In a few clinical studies on fatigue and other symptoms, the effects of MLR GPL supplements on gastrointestinal symptoms were also examined.146,159 For example, in a study on fatigue, pain, gastrointestinal, and other symptoms in fibromyalgia patients taking 4.8 g of MLR GPL/day, there were significant (p < 0.001) reductions in these and other symptoms, as well as improvements in overall symptom severities and quality of life. Regression analysis of the data demonstrated that the reductions in symptom severities were consistent and occurred with a low degree of variance.146 In chemically-exposed veterans (average age 53) who took 6 g/day MLR GPL for six months, there were significant reductions in a wide variety of symptoms. The reductions in symptom severities were found to be gradual and also had a low degree of variance.159 This study will also be discussed further in a subsequent section on chemical detoxification.
MLR supplements can also reduce the gastrointestinal side effects of commonly used non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs, like aspirin or ibuprofen, can reduce mucus production in the epithelial cell lining. MLR supplements have been used to increase the production of gastrointestinal mucus. For example, a soy MLR GPL product has been used to reduce the severity of gastric mucosal lesions produced by NSAID use. Supplementation with this MLR GPL product for 14 days was shown to help heal ulcers and decrease abdominal pain.179 Thus, in addition to preventing the loss of mucus in the gut epithelial lining, MLR GPL supplements can aid in repairing damaged epithelial membranes and the membranes of surrounding cells.
Cancer and neoplastic diseases
The use of MLR supplements for clinical cancer support, such as cancer-related fatigue and pain, and to help reduce the adverse effects of chemotherapy and radiotherapy, are discussed elsewhere.180,181 Briefly, anti-cancer therapy, such as chemotherapy and radiotherapy, results in the generation of excess ROS, and this excess oxidative stress can damage cellular membranes and cause adverse symptoms. For example, during therapy, ROS are generated by anthracycline antibiotics, alkylating agents, other drugs, and radiotherapy.156,180,181 Chemotherapy can also displace important mitochondrial cofactors from MIM, such as CoQ10.180
MLR supplementation has been used to diminish the adverse effects of chemotherapy (and potentially radiotherapy) in cancer patients, especially those of advanced age. For example, an MLR GPL product has been utilized in cancer patients to reduce some of the most common adverse effects of cancer therapy, such as chemotherapy-induced cancer-related fatigue, malaise, headaches, nausea, vomiting, diarrhea, and other side effects.180,181 In two clinical studies using advanced metastatic colon, pancreatic, or rectal cancer patients who received combination chemotherapy, MLR was used to reduce the adverse effects of chemotherapy.182 When patients used the MLR GPL supplement, there were significantly fewer episodes of fatigue, insomnia, nausea, diarrhea, constipation, skin changes, and other adverse effects compared to the control chemotherapy group. Adding MLR phospholipids to post-cancer therapy also resulted in improvements in the incidence of fatigue, insomnia, nausea, diarrhea, impaired taste, constipation, and quality of life indicators.182
Cardiovascular conditions
Long-term oral ingestion of MLR phospholipids in aging adults can produce blood lipid profiles that are associated with a reduced risk of certain chronic morbidities, such as those seen in cardiovascular diseases (reviewed in Ref. 107). For example, blood cholesterol levels were lowered significantly in patients with hyperlipidemia who used MLR GPL supplements.183,184 In one study, diabetic patients received MLR GPL treatment for two months, and this resulted in lower total blood cholesterol, low-density lipoproteins (LDL) cholesterol, and triglycerides, while high-density lipoproteins cholesterol was increased.185 These are lipid profiles that are consistent with a reduced risk for cardiovascular disease.109,110
Hypertension, another risk factor for cardiovascular disease that is commonly found in aging populations, has also been attenuated with MLR phospholipid supplementation. In studies with obese patients with hypertension, dietary GPL reduced total blood cholesterol, LDL, and other lipids while reducing blood pressure (reviewed in Ref. 109).
High blood levels of apolipoprotein A1 (apoA1) have been proposed to be protective against arteriosclerotic disease. Therefore, Poliche et al.186 used PC supplementation to increase the blood levels of apoA1 in patients with type IIA hypercholesterolemia. Poliche et al.186 found that MLR PC significantly increased the blood levels of apoA1 and apolipoprotein E and thus are likely to contribute to a reduced risk of arteriosclerosis.186
A blood marker that has been especially useful for predicting hospitalization and death from heart failure is homocysteine. High homocysteine blood levels are associated with an increased risk of hospitalization and death due to heart failure, stroke, renal failure, and other medical conditions.187,188 Patients greater than 60 years of age with blood homocysteine in the high-risk concentration range were given a combination MLR GPL supplement to reduce their chronic fatigue, and as part of their routine clinical tests, blood homocysteine levels were followed for six months.150 In these patients, there was an average reduction in blood homocysteine levels of 31.8% (p < 0.001) by the end of the monitoring period, and these lower levels were consistently below the blood levels of homocysteine that predict cardiovascular adverse events. After six months on the MLR supplement, their blood homocysteine levels were reduced from high-risk levels (mean, 10.85 ± 0.42 µmole/L) to blood levels (mean, 7.40 ± 0.42 µmol/L) that do not predict hospitalization and death due to heart disease. There was also a 59.6% reduction in fasting insulin levels, and there were no cardiovascular events during the trial and for at least six months after completion of the trial.150 Similar results were found with an MLR preparation by Olthof et al.189 in healthy aged men. In this study, homocysteine blood levels, along with total cholesterol, LDL, and triglycerides, were reduced significantly within 30 days.
Chronic inflammation
Chronic inflammation has been implicated in many diseases and morbidities, including various types of cancer, heart disease, Alzheimer’s disease, asthma, rheumatoid arthritis, and type 2 diabetes.190 Chronic inflammation is a common condition in advanced age and a significant risk factor for morbidity and mortality among the elderly.191 In seniors, it is characterized by the presence of excess pro-inflammatory cytokines that can negatively impact the body internally, affecting multiple organs and tissues while showing no initial outward symptoms.23,192 Such inflammation can be amplified by several factors, including diet, smoking, excessive consumption of alcohol, stress, weight gain, reduced physical activity, and sleep disturbances.193 High blood levels of pro-inflammatory markers and their presence in other tissues are often found in older individuals who also have reduced physical and cognitive functions. The presence of high levels of pro-inflammatory cytokines has been shown to increase the risk of cardiovascular disease, frailty, and other morbidities.23,192
Chronic inflammation in seniors, also termed inflamm-aging, is related to the activation of innate immunity, excess chronic pro-inflammation, and other factors.192–194 Inflamm-aging is usually managed by changes in lifestyle and diet. For example, modifications of diet to include caloric restriction and the intake of foods rich in antioxidants and polyphenols, as well as dietary MLR supplements, have been used to reduce inflamm-aging.195,196 Using a marine-sourced GPL with a high content of n-3 FA, reductions in inflammatory reactions were assessed in older patients with cardiovascular disease and/or rheumatoid arthritis by the inhibition of inflammatory prostaglandins. Supplementation with the krill oil preparation (300 mg/day) reduced C-reactive protein levels and moderated the severity of several symptoms, such as pain, joint stiffness, and other symptoms.197 Krill oil has also been used to reduce various symptoms associated with inflammation, such as premenstrual syndrome. In a blinded, randomized clinical trial, a krill oil supplement was found to be effective in reducing premenstrual syndrome symptoms, including joint pain, abdominal pain, swelling, and breast tenderness.198 In another study, Hirose et al.147 used 0.6 or 1.2 g/day MLR GPL to reduce symptom severity in aging women with menopausal symptoms. This randomized, double-blind, placebo-controlled trial found higher mood and quality of life scores with the higher MLR daily dose, as well as reductions in diastolic blood pressure and cardio-ankle vascular index (p = 0.03–0.05).147
Chemical detoxification
As described above, oral MLR can be used to lower and remove excess blood cholesterol as well as oxidized, damaged GPL and reduce the risk of cardiovascular disease.183,184,186 This process appears to work through a “mass action” or “bulk flow” mechanism that is concentration-dependent.122 By administering higher doses of MLR GPL (usually 5–6 g/day), this process can be used to safely remove toxic, hydrophobic molecules from cells and tissues. The removal process is thought to work by the partitioning and exchange of hydrophobic toxins, petrochemicals, and other toxic hydrophobic molecules into lipid globules, liposomes, chylomicrons, and other lipid structures that form when excess phospholipids are present due to the dietary influx of MLR lipids.110,120,122 By partitioning, exchanging, and secreting the damaged lipids (and hydrophobic chemicals and toxins), they can be slowly transferred from cells and tissues into the blood circulation and eventually delivered to the small intestinal epithelium, where further exchange can occur with gastrointestinal lipid globules and liposomes. More simply, the lipid forms are more directly expelled in a concentration-driven process. Thus, the “conveyor belt” process can remove excess damaged lipids and other hydrophobic toxic molecules and chemicals, which are eventually secreted in the stool (Fig. 3).110,123,159
Using obese and/or diabetic patients, the use of MLR lipids, such as Essentiale® phospholipids, has been found useful for the treatment of patients with non-alcoholic fatty liver disease or alcoholic liver disease. Readers are referred to the comprehensive reviews by Gundermann et al. for more details.152,153 These clinical studies used MLR lipids at IV doses ranging from 1.05–1.8 g/day, and the treatments lasted from one to 24 months. The outcomes of these studies depended on clinical assessments, including the reduction of pain, hepatomegaly, dyspeptic symptoms, etc., and were supported by various imaging procedures, histology, and lab tests for markers of liver cytolysis. Gundermann et al.153 have stressed that the success of the MLR GPL in treating liver diseases depended on its high content of PC (72–96% PC, especially 1,2-dilinoleoylphosphatidylcholine) to increase the general fluidity and repair of liver cell membranes and provide anti-inflammatory, anti-fibrotic, apoptosis-, cell receptor-, and lipid signal-regulating properties.
The likely reason that the above approaches were successful is based, in part, on the principles of “bulk flow” or “mass action” exchange of MLR lipids.122 As described above, this approach has also been used to treat chemically exposed veterans and reduce a variety of symptom severities.159 In this clinical study, veterans were exposed to burn pits, oil well fires, and other environmental sources of chemical contaminants, and they later presented with multi-symptom illnesses (Gulf War Illnesses). Even two decades after exposures, their symptoms persisted, so these veterans were placed on 6 g/day oral MLR GPL for six months, and their multi-symptom severities were self-reported over this time period. During this open-label clinical trial, there were gradual and significant reductions in symptom severities in several categories related to fatigue, pain, breathing, vision, sleep, balance, urinary and gastrointestinal symptoms, musculoskeletal and nasopharyngeal symptoms, and chemical sensitivities. There were no adverse incidents during the study, and the all-natural oral study MLR supplement was extremely well tolerated.159 In the future, the use of MLR supplements to remove or counteract hydrophobic toxins or other toxic chemicals should be considered for the treatment of chronic illnesses where environmental hydrophobic toxins or contaminants are present.
MLR dose levels in clinical studies
MLR GPL supplements have been used in various daily dose levels to reduce signs and symptoms in various clinical conditions (Table 2). In earlier clinical studies, there was an attempt to find daily MLR dose levels that resulted in positive outcomes, for example, in enhancing mitochondrial function or reducing fatigue. We have used increasing daily dose levels of MLR GPL to improve results and significantly decrease various symptom severities, but this still often required several months of treatment to observe significant differences. To speed up the process of reducing symptom severities, we have gradually increased the daily dose levels of MLR GPL in clinical studies to improve the rates of turnover, exchange, and replacement of damaged membrane lipids.158 Our goal has been to repair enough damage to cellular membranes in a timely manner to return membrane-associated functions to the levels seen in normal age-matched subjects.
Some clinical conditions apparently require higher threshold doses of MLR lipids to be effective compared to those employed for general nutritional purposes. Although the lower MLR GPL dose levels (for example, 1 g/day MLR GPL) were found to be useful in achieving positive results in reducing some symptoms over time, we have found that higher dose levels (for example, 4–6 g/day or higher) resulted in significant reductions in symptom severities within weeks instead of months. An example of this is the reduction of pain by MLR phospholipids. The reduction of widespread pain required a threshold level or a much higher daily dose level of MLR lipids (>5g/day GPL) to see any benefits.158,159 The possible reason for the requirement of higher doses of MLR phospholipids to see significant reductions in widespread pain could be due to the effects of a very small fraction of a specific GPL in MLR supplements. We reasoned that it could be a minor GPL fraction in an MLR supplement that bound to and stabilized nerve membrane channels, normalized pain, and affected neuron cells in terms of resting potentials and the spontaneous triggering of depolarization.40 Recent results using 6 g/day MLR GPL to drive the replacement or removal of damaged lipids and toxic hydrophobic chemicals in chemically exposed veterans further confirmed that this process is likely driven by a mass-action mechanism (as proposed by Mayor et al.122) that works best when higher daily dose levels of MLR lipids are employed.159
Conclusions and perspective
Finding suitable, safe, and effective anti-aging supplements for an aging populace has been an ongoing goal in the nutraceutical field. Here we have described an approach to developing new, safe nutraceuticals with beneficial uses for human health based on basic scientific information, such as the composition, structure, and dynamics of biological membranes and the activities of their components. In the example here, MLR supplements that contain inulin-protected, undamaged membrane GPL have been found to be effective in a number of clinical studies in improving health. Efforts have been made with MLR to improve and lower age-associated symptoms and increase quality of life indicators. MLR supplementation also appears to be useful for delaying some of the functional decline seen in patients as they age by restoring cellular functions to levels found in younger adults, such as increasing mitochondrial function. Thus, MLR supplementation has been especially useful for safely reducing certain age-related morbidities, such as those found in cardiovascular conditions, neurodegenerative diseases, chronic infections, and metabolic disorders. In addition, MLR with GPL can modify a variety of general symptoms like age-associated fatigue and frailty and improve quality of life indicators. MLR supplements also have other uses; for example, they have been shown to improve the uptake and bioavailability of some nutrients and cofactors. Finally, MLR supplementation can be used to slowly remove hydrophobic toxins and other toxic molecules, such as petrochemical contaminants, which can accumulate with age and often accompany environmental exposures and long-term chronic conditions.
Declarations
Acknowledgement
The authors acknowledge the assistance of John Michael for preparing the illustrations.
Funding
GLN acknowledges support from the Institute for Molecular Medicine and Nutritional Therapeutics, Inc. GFM has support from Research and Development Funds CSIC 91,137 and p22520220100007UD 2022 from Universidad de la República.
Conflict of interest
GLN is a part-time consultant to Naturally Plus Taiwan and Nutritional Therapeutics, Inc. RS is a part-time consultant to Naturally Plus USA and Nutritional Therapeutics, Inc. GF and PB have no conflicts to disclose.
Authors’ contributions
Conceptualization, original draft preparation, revision (GLN), section additions (GF), review, and editing (GLN, GF, RS, PB).

 Author information
Author information