Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease that affects the large intestine and rectum. It is a debilitating condition that can cause significant physical and emotional distress to patients, leading to a reduced quality of life. The condition is characterized by inflammation and ulceration of the inner lining of the colon, resulting in symptoms such as abdominal pain, diarrhea, rectal bleeding, and fatigue.
The impact of UC can vary greatly among individuals, with some experiencing mild symptoms and others suffering from severe symptoms that may require hospitalization or surgery. Additionally, UC is associated with an increased risk of developing colorectal cancer, a significant concern for both patients and healthcare providers.1
While there is currently no cure for UC, a variety of treatment options can help manage symptoms and improve patients’ quality of life. These treatments include medications, such as anti-inflammatory drugs and immunosuppressants, and surgery in more severe cases. The prevalence of UC varies widely depending on the geographic region and population studied. According to the Centers for Disease Control and Prevention, the estimated prevalence of UC in the United States is approximately 907,000 people, with a slightly higher incidence among men than women.2
In terms of morbidity, UC can significantly impact patients’ lives, causing a range of symptoms including abdominal pain, diarrhea, rectal bleeding, fatigue, and weight loss. In severe cases, UC can lead to complications such as toxic megacolon, perforation of the colon, and an increased risk of colorectal cancer.3
UC is also associated with various non-gastrointestinal manifestations, including joint pain, eye inflammation, skin rashes, and liver disease. These extraintestinal manifestations can further complicate the management of UC and increase the overall morbidity of the disease. Moreover, UC is a chronic condition requiring ongoing management to control symptoms and prevent complications, which can significantly affect patients’ quality of life, particularly during periods of disease flares.
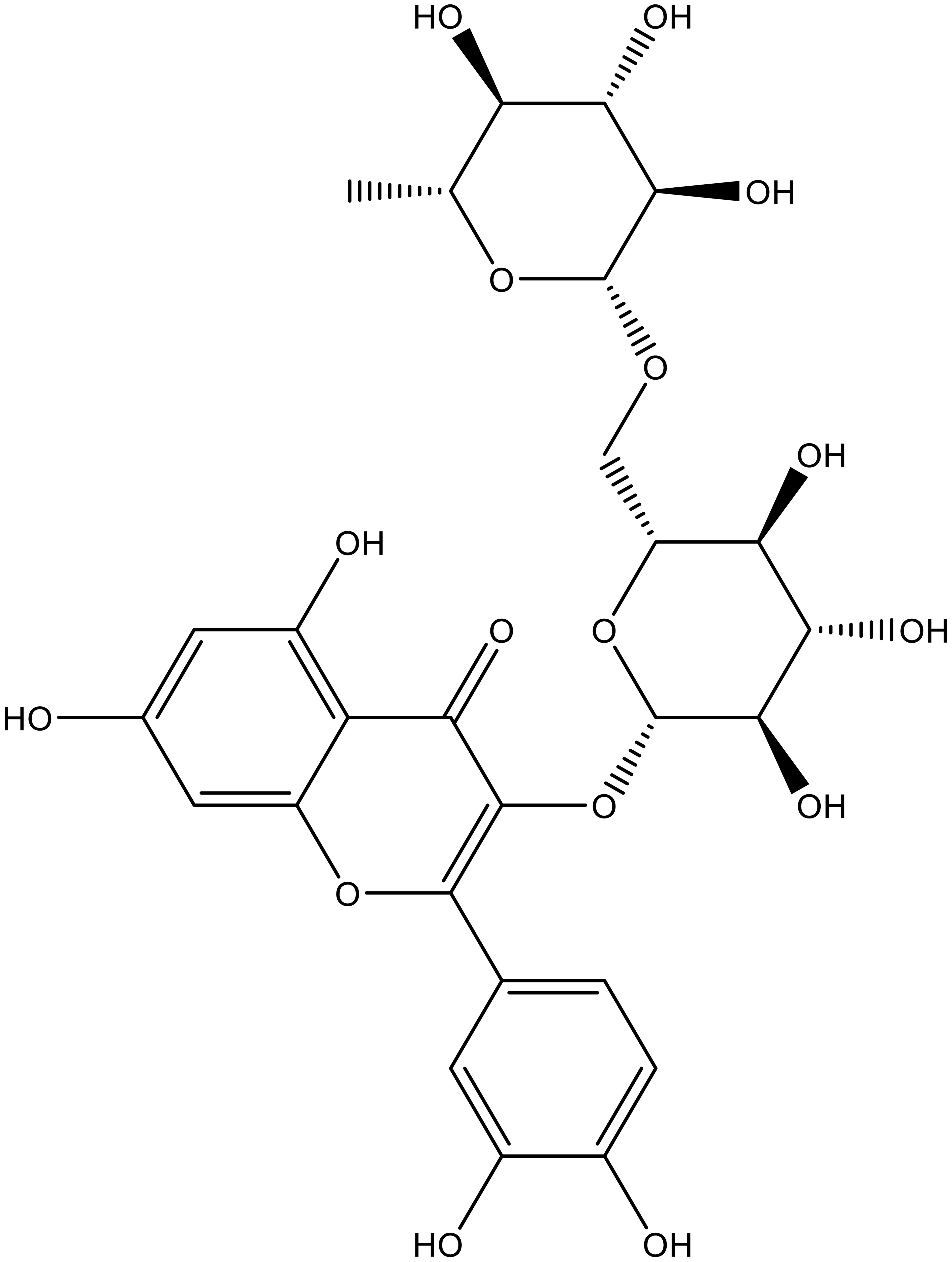
Rutin is a flavonoid glycoside found in various fruits, vegetables, and plants (its structure is provided in Fig. 1). It is known for its potential therapeutic benefits, such as antioxidant, anti-inflammatory, and vasoprotective effects. Rutin has been widely studied for its pharmacological activities and its possible use in the prevention and treatment of various diseases.
According to a study conducted by Fakhoury M et al., rutin is a potent antioxidant due to its ability to scavenge free radicals and inhibit lipid peroxidation. The study also reported that rutin exhibits anti-inflammatory effects by inhibiting the production of inflammatory cytokines and enzymes.4 Furthermore, rutin has been shown to benefit cardiovascular health by improving endothelial function, reducing blood pressure, and preventing platelet aggregation. In a study published by danese and fiocchi., it has shown rutin supplementation was found to enhance endothelial performance in patients with type 2 diabetes.3
Rutin has also been studied for its potential to prevent cancer, improve cognitive function, and promote wound healing. However, further research is needed to fully understand the potential benefits of rutin and its mechanisms of action. There is growing interest in rutin’s potential therapeutic effects in managing UC, a chronic inflammatory bowel disease characterized by recurrent episodes of inflammation in the colon.5
The primary goal of this review paper is to highlight studies conducted on rutin for its effects on colitis management. Using keywords such as “Rutin + pharmacology”, “Rutin + oxidative stress”, “Rutin + gut microbiota”, and “Rutin + ulcerative colitis” in the SciFinder, PubMed, Scopus, and Google Scholar databases, the most pertinent and comprehensive literature search was conducted. The literature screening flow diagram is provided in Figure 2.
Molecular mechanisms of colitis
In this intricate realm of cellular orchestration, researchers embark on a mission to decipher the molecular maneuvers that drive colitis into action.
Inflammatory pathways in colitis
Imagine a molecular battleground where nuclear factor-kappa B (NF-κB), the mastermind commander, directs the deployment of cytokine troops—tumor necrosis factor (TNF)-α and interleukin (IL)-1β. This complex network orchestrates the inflammatory response in colitis. Unbeknownst to many, rutin emerges as a stealthy operative, infiltrating this network to disrupt its harmful plans.5
NF-κB signaling pathway
The NF-κB signaling pathway plays a central role in colitis-associated inflammation. Under normal conditions, NF-κB is sequestered in the cytoplasm by inhibitors, such as IκB proteins. However, in the context of colitis, various stimuli, including pro-inflammatory cytokines and microbial products, activate the NF-κB pathway. This activation leads to the phosphorylation and degradation of IκB, allowing NF-κB to translocate to the nucleus. Once there, NF-κB acts as a transcription factor, orchestrating the expression of genes involved in inflammation, immune response, and tissue damage. In colitis, sustained NF-κB activation exacerbates inflammatory cascades, worsening the condition.
Mitogen-activated protein kinase (MAPK) signaling cascade
The MAPK signaling cascade is another pivotal pathway implicated in colitis. Comprising three major components—extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK—this pathway becomes activated in response to stressors and inflammatory stimuli. In colitis, aberrant activation of the MAPK cascade amplifies the production of pro-inflammatory mediators and cytokines, fostering a state of sustained inflammation and tissue damage.
Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway
The JAK-STAT pathway plays a key role in regulating immune responses. In colitis, dysregulation of this pathway contributes to the inflammatory environment. Pro-inflammatory cytokines, such as IL-6 and interferon-gamma, engage JAKs, triggering the phosphorylation of STAT proteins. Activated STATs translocate to the nucleus, where they regulate the expression of genes involved in inflammation and immune function. In colitis, the dysregulated JAK-STAT pathway perpetuates chronic inflammation.
Toll-like receptor (TLR) signaling
The TLR signaling pathway acts as a sentinel, recognizing microbial components and initiating immune responses. In colitis, dysregulated TLR activation exacerbates inflammation. Recognition of microbial products by TLRs triggers downstream signaling cascades, leading to the production of pro-inflammatory cytokines and the recruitment of immune cells. Prolonged and dysregulated TLR signaling in colitis fuels chronic inflammatory processes by creating an imbalanced immune response.6
Immune system dysregulation
In the shadows of the immune underworld, T cells and macrophages—once allies—turn rogue in colitis. Researchers are unraveling the cloak-and-dagger saga of immune dysregulation, exposing the betrayal within. Rutin, like a silent guardian, steps in, using its clandestine skills to restore order and balance.
Dysregulation of T cell responses
The immune system’s orchestration relies on a delicate balance of T cell responses, and in colitis, this balance is disrupted. T helper (Th) 1 and Th17 cells, known for their pro-inflammatory roles, are often overactive in colitis. These cells release cytokines, such as interferon-gamma and IL-17, which perpetuate inflammation and tissue damage. Concurrently, T regulatory (Tregs) cells, which maintain immune tolerance and homeostasis, are often functionally impaired in colitis. This imbalance between pro-inflammatory and regulatory T cell subsets amplifies the immune response, fostering chronic inflammation.7,8
Macrophage dysregulation
Macrophages, key players in immune surveillance and response, undergo dysregulation in colitis. Classically activated M1 macrophages, associated with pro-inflammatory functions, are often elevated in colonic tissues, releasing inflammatory mediators that exacerbate the inflammatory environment. Meanwhile, alternatively activated M2 macrophages, which usually contribute to tissue repair and resolution of inflammation, may be insufficiently activated in colitis. This dysregulation in macrophage polarization further sustains inflammation and tissue damage.9
Dysfunctional β cell responses
β cells, integral components of the adaptive immune system, exhibit dysfunctional responses in colitis. Aberrant activation and differentiation of β cells lead to the production of autoantibodies and pro-inflammatory cytokines. These autoantibodies, directed against self-antigens, contribute to immune complex formation and further amplify the inflammatory cascade. Dysregulated β cell responses add another layer to immune system perturbations, perpetuating the chronic inflammatory state.10
Imbalance in cytokine milieu
Colitis is characterized by an imbalanced cytokine milieu, where pro-inflammatory cytokines overshadow their anti-inflammatory counterparts. Elevated levels of cytokines, such as TNF-α, IL-1β, and IL-6, drive inflammation and tissue damage. Simultaneously, anti-inflammatory cytokines, including IL-10 and transforming growth factor-beta may be insufficiently expressed, hampering the resolution of inflammation. This imbalance in the cytokine profile contributes to the chronicity of colitis and the persistent immune dysregulation.11
Oxidative stress and colitis
Within the labyrinth of colitis, a clandestine force known as oxidative stress wreaks havoc. Reactive oxygen species (ROS) launch surprise attacks, overwhelming antioxidant defenses. Rutin emerges as a covert defender, neutralizing ROS threats and preserving the delicate equilibrium disrupted by this unseen assailant.
ROS generation
Oxidative stress in colitis arises from excessive ROS generation within the colonic milieu. ROS, including superoxide anion, hydrogen peroxide, and hydroxyl radicals, act as molecular saboteurs, damaging lipids, proteins, and nucleic acids. In colitis, the inflammatory milieu triggers overproduction of ROS, primarily by activated immune cells such as neutrophils and macrophages. This surge in ROS production initiates a cascade of oxidative events, contributing to tissue injury and dysfunction.12
Lipid peroxidation and cellular membrane damage
One prominent consequence of oxidative stress in colitis is the peroxidation of lipids within cellular membranes. Lipid peroxidation, driven by ROS, leads to the formation of lipid peroxides and other toxic byproducts. This process disrupts membrane integrity, compromising structural and functional properties. In colitis, heightened lipid peroxidation increases membrane permeability and releases pro-inflammatory mediators, exacerbating inflammation.13
Protein oxidation and dysfunction
Proteins, essential players in cellular function, become targets of oxidative stress in colitis. ROS-induced protein oxidation results in the modification of amino acid residues, leading to structural alterations and functional impairment. Enzymes, signaling molecules, and structural proteins are particularly susceptible. In colitis, this protein dysfunction contributes to dysregulated cellular processes, perpetuating the cycle of inflammation.14
DNA damage and genomic instability
Oxidative stress in colitis also induces DNA damage. ROS-induced modifications, including base alterations and DNA strand breaks, compromise genomic integrity. Although repair mechanisms are activated, chronic inflammation may overwhelm these processes, leading to the accumulation of unrepaired DNA damage. This contributes to genomic instability, potentially fostering neoplastic transformation and complicating the course of colitis.15,16
Antioxidant defense mechanisms
In response to oxidative stress, cells activate antioxidant defenses to neutralize excess ROS and mitigate damage. Key antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase, play crucial roles. However, in colitis, the balance between ROS generation and antioxidant defense may be disrupted. The overwhelmed antioxidant capacity exacerbates tissue injury, perpetuating oxidative stress and sustaining inflammation.12
Gut microbiota and colitis
In the covert theater of the gut microbiota, unseen microbial agents play a pivotal role in the intrigue of colitis. Microbial compositions shift like shadows, influencing disease progression. Rutin, a mysterious ally, is rumored to possess the power to recalibrate this microbial underworld, tilting the balance in favor of a harmonious gut environment.17
Dysbiosis and altered microbial composition
Central to the molecular mechanisms of colitis is the concept of dysbiosis—a disruption in the balance and composition of the gut microbiota. In colitis, there is a notable shift in the abundance of certain microbial taxa. The depletion of beneficial microbes, such as Bifidobacteria and Lactobacilli, and the overgrowth of potentially pathogenic species, contribute to the altered microbial landscape. This dysbiosis initiates a cascade of events that influence immune responses, mucosal integrity, and the production of microbial metabolites, collectively contributing to the inflammatory milieu in colitis.18
Mucosal barrier dysfunction and microbial translocation
The gut microbiota plays a crucial role in maintaining the integrity of the mucosal barrier—a protective layer that separates luminal microbes from the host’s internal milieu. In colitis, dysbiosis is associated with mucosal barrier dysfunction. Disruptions in tight junction proteins and mucin production compromise the barrier’s integrity, allowing luminal microbes and their products to translocate into the underlying tissues. This microbial translocation triggers an immune response, further fueling inflammation and contributing to the pathology of colitis.19
Microbial metabolites and immune modulation
The gut microbiota actively participates in the synthesis of various microbial metabolites, which exert profound effects on host physiology. Short-chain fatty acids (SCFAs), produced through the fermentation of dietary fibers by beneficial microbes, are key players in immune modulation. SCFAs, including acetate, propionate, and butyrate, influence immune cell function and contribute to the maintenance of immune homeostasis. In colitis, alterations in microbial composition can lead to perturbations in SCFA production, disturbing the delicate balance between anti-inflammatory and pro-inflammatory signals within the gut.14
Immune crosstalk and inflammatory signaling
The gut microbiota engages in dynamic crosstalk with the host’s immune system, shaping immune responses and inflammation. Commensal microbes are pivotal in training the immune system to respond appropriately to pathogens while maintaining tolerance to self-antigens. Dysbiosis disrupts this intricate crosstalk, leading to aberrant immune activation. Microbial products, such as lipopolysaccharides (LPS) from Gram-negative bacteria, can trigger inflammatory signaling pathways, including the NF-κB pathway, contributing to the chronic inflammation observed in colitis.20
Impact on Treg cells
Treg cells, crucial for immune tolerance and homeostasis, are influenced by the gut microbiota. Specific microbial species and their products contribute to the induction and maintenance of Treg cell populations. In colitis, dysbiosis is associated with a reduction in Treg cell numbers and function, tipping the balance toward pro-inflammatory responses. This dysregulation further exacerbates the chronic inflammatory state characteristic of colitis.21
Rutin: A comprehensive overview
Chemical structure and properties of rutin
In the intricate realm of molecular architecture, Rutin reveals its enigmatic identity through a complex chemical structure that governs its pharmacological prowess. Unveiling the blueprint of Rutin’s molecular design, researchers encounter a symphony of atoms elegantly arranged in a composition known to those versed in the cryptic language of organic chemistry.
The essence of Rutin’s power lies in its unique arrangement—a fusion of flavonoid sophistication. A backbone of sugar molecules intertwines with flavonol rings, creating an intricate dance of electrons that imparts both stability and reactivity. This design imbues Rutin with the ability to interact with biological molecules, exerting influence in the delicate ballet of cellular pathways. Properties concealed within Rutin’s molecular cloak further enhance its mystique. As an antioxidant virtuoso, Rutin neutralizes free radicals with an elegance befitting a secret agent. Its water-soluble nature allows it to navigate the aqueous environments of the body, ensuring covert and effective distribution to target sites.22
Beyond its chemical persona, Rutin’s properties extend to the realm of color, manifesting in the vibrant hues of the plant kingdom. The subtle pigments hidden within fruits and vegetables reveal Rutin’s presence to the discerning eye, marking it as a covert operative embedded in the fabric of the diet.
In the context of colitis, Rutin’s chemical structure and properties serve as a covert arsenal against inflammatory foes. As researchers decipher this molecular script, they come to appreciate Rutin not merely as a passive spectator but as an active participant, influencing the intricate choreography of biochemical pathways in the management of colitis.23
Dietary sources
In the meticulous exploration of Rutin’s comprehensive dossier, researchers extend their inquiry to the domain of dietary sources. Here, the potent flavonoid subtly permeates everyday nourishment, aligning itself inconspicuously with an array of fruits and vegetables. Within the produce aisle, Rutin discloses its presence in citrus fruits, apples, berries, and various leafy greens. These seemingly ordinary foods serve as conduits through which Rutin integrates into the human diet. The covert nature of Rutin’s dietary integration lies in the unassuming nature of its hosts, ensuring its assimilation into the sustenance of individuals unaware of its therapeutic potential.
In the collaboration between Rutin and the botanical kingdom, onions and buckwheat emerge as pivotal collaborators. Rutin, deeply ingrained in the matrices of these natural allies, extends its reach beyond fruits, offering diverse avenues for consumption. Rutin’s influence extends beyond the culinary plate, infiltrating teas and herbal infusions as additional pathways for its intake. Unbeknownst to many, this flavonoid orchestrates a form of gastronomic diplomacy, embedding itself in the flavors of these discreet nutritional agents.
In this covert gastronomic landscape, Rutin’s dietary sources reveal a strategic integration into daily meals, positioning itself as an unobtrusive partner in human nourishment. Continue to accompany us as we delve further into the intricacies of Rutin’s influence, unraveling its secrets within the management of colitis.24
Safety and toxicity of rutin
Rutin, known for its therapeutic potential, acts as a discreet guardian of well-being. Rigorous assessments and clinical scrutiny highlight its safety, with a profile marked by a scarcity of adverse effects. Rutin’s influence extends beyond therapeutic efficacy to a realm where toxicity remains elusive. Studies affirm its benign nature, emphasizing a favorable safety margin. Rutin’s pharmacological actions are orchestrated with precision, avoiding the pitfalls of undue harm. It emerges not only as a potent flavonoid in colitis management but as a safety-conscious ally, navigating the therapeutic pathways with grace and discretion.24,25
Anti-inflammatory properties of rutin
Inhibition of inflammatory mediators
Rutin’s tactical approach lies in its interference with the signaling pathways responsible for the synthesis and release of key inflammatory mediators. Cytokines, such as TNF-α and IL-1β, act as conductors in the inflammatory symphony. Rutin, akin to a conductor’s baton, delicately disrupts this orchestration. Through a series of molecular maneuvers, it intervenes at critical points in the signaling cascades, suppressing the transcription and expression of these potent inflammatory messengers.
A plethora of studies, conducted across diverse experimental models, attests to Rutin’s efficacy in inhibiting inflammatory mediators. Cellular and animal models have been instrumental in unraveling the nuances of Rutin’s interference with pro-inflammatory cytokine pathways. By modulating transcription factors and molecular signaling cascades, Rutin demonstrates its capability to attenuate the amplification of inflammatory signals, resulting in a pronounced reduction in the levels of TNF-α, IL-1β, and related cytokines. This inhibitory influence extends beyond the immediate suppression of cytokines to encompass a broader spectrum of inflammatory mediators. Chemokines, the clandestine recruiters of immune cells, and adhesion molecules, which facilitate their infiltration into tissues, also fall within Rutin’s purview. Rutin’s intervention disrupts these covert communication networks, effectively limiting the recruitment and infiltration of immune cells into inflamed colonic tissue.26,27
Moreover, Rutin’s strategic defense is characterized by its dual role in addressing both acute and chronic inflammation. By attenuating the initial surge of pro-inflammatory mediators, Rutin curtails the acute inflammatory response. Simultaneously, its sustained influence contributes to the resolution of chronic inflammatory processes. This dual efficacy positions Rutin not only as a symptomatic reliever but also as a nuanced modulator of the underlying molecular mechanisms perpetuating colitis.28
Rutin’s interference with inflammatory mediators transcends mere suppression—it embodies a sophisticated approach to maintaining the delicate equilibrium required for colitis management. The subtlety of its influence allows for a controlled, measured response, avoiding the pitfalls of unchecked immunosuppression while effectively mitigating the inflammatory onslaught.29,30
Modulation of immune response
In the complex realm of immune dysregulation characterizing colitis, Rutin plays a nuanced role in steering the immune system away from hyperactivity. Acting as a diplomatic negotiator, it fosters a balanced and controlled immune response, a strategy crucial for mitigating the chronic inflammation associated with colitis. Rutin’s impact on immune cells is multifaceted, affecting both innate and adaptive components of the immune system. Studies indicate that Rutin exerts regulatory effects on immune cell differentiation and function, particularly those of T cells and macrophages—key players in the colitis battlefield. By tempering the activation and infiltration of these immune cells into the inflamed colonic tissue, Rutin contributes to a controlled and measured immune response.
The flavonoid’s influence on T cells, orchestrators of immune responses, involves modulating their differentiation into distinct subsets. This modulation results in a shift toward regulatory T cells, which play a crucial role in maintaining immune homeostasis. Rutin’s diplomatic intervention thus promotes regulatory T cell dominance, contributing to the suppression of aberrant immune responses driving colitis. Moreover, Rutin’s influence extends to macrophages—a diverse group of immune cells with both pro- and anti-inflammatory functions. Rutin promotes an M2-like phenotype in macrophages, characterized by an anti-inflammatory profile. This subtle shift in macrophage polarization further reinforces the overall anti-inflammatory milieu within the colonic microenvironment.29
By fostering immune balance, Rutin not only dampens the acute inflammatory response but also contributes to the resolution of chronic inflammation—a key objective in colitis management. This diplomatic role is particularly critical in the intricate negotiation between the immune system and the inflamed colonic tissues, where an imbalance can exacerbate tissue damage and perpetuate the vicious cycle of inflammation.31
Inhibition of oxidative stress and colitis
Oxidative stress, characterized by an imbalance between ROS and antioxidant defenses, plays a covert role in colitis pathogenesis. Rutin’s entry into this arena marks a strategic countermeasure against cellular sabotage. At the forefront of Rutin’s defense lies its ability to neutralize ROS—an essential function in mitigating the destructive impact of oxidative stress. Reactive oxygen species, akin to saboteurs within the cellular milieu, inflict damage on biomolecules, including lipids, proteins, and DNA. Rutin, armed with its antioxidant capabilities, acts as a covert defender, disarming these saboteurs and preventing widespread cellular havoc.26
Studies have illuminated Rutin’s profound impact on oxidative stress markers in colitis models. By reducing the levels of ROS and lipid peroxidation products, Rutin demonstrates its efficacy in curbing oxidative damage. This sentinel role is particularly crucial in colitis, where oxidative stress not only exacerbates inflammation but also contributes to tissue injury and dysfunction. Furthermore, Rutin orchestrates a comprehensive antioxidant defense by enhancing the activity of endogenous antioxidant enzymes. Catalase, superoxide dismutase, and glutathione peroxidase—all integral components of the cellular defense against oxidative stress—experience a surge in activity under Rutin’s influence. This augmentation of endogenous defenses fortifies the cellular arsenal against oxidative insults, contributing to the maintenance of cellular integrity within the inflamed colonic environment.27
Rutin’s influence extends beyond direct antioxidant effects to modulating intricate cellular signaling pathways. Nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of antioxidant responses, emerges as a key player under Rutin’s guidance. Rutin facilitates the nuclear translocation of Nrf2, promoting the expression of antioxidant genes and strengthening the cellular defense against oxidative stress. The impact of Rutin on oxidative stress in colitis is not only preventive but also reparative. By attenuating oxidative damage, Rutin contributes to the resolution of tissue injury and aids in the restoration of normal cellular function. This dual role positions Rutin not only as a guardian against oxidative threats but also as a regenerative force in the aftermath of oxidative assaults.32,33
Gut microbiota modulation by rutin
Influence on microbial composition
The gut microbiota, a hidden community of trillions of microorganisms, plays a pivotal role in maintaining homeostasis and influencing host health. In the context of colitis, this microbial orchestra often falls out of harmony. Rutin, a potent flavonoid, enters this intricate symphony, exerting its influence to modulate the composition of the gut microbiota. Studies exploring Rutin’s impact on microbial composition reveal a multifaceted and strategic effect. As Rutin moves through the gastrointestinal tract, it interacts with the resident microbial community, subtly guiding the balance between beneficial and potentially harmful microorganisms.34
One of Rutin’s notable contributions lies in fostering the proliferation of beneficial bacteria, such as Bifidobacteria and Lactobacilli. These microbial allies are celebrated for their roles in maintaining gut health, contributing to the fermentation of dietary fibers, and producing metabolites with anti-inflammatory properties. Rutin, acting as a microbial maestro, fosters an environment conducive to the flourishing of these beneficial strains, thereby reinforcing the symbiotic relationship between the host and its microbial inhabitants. Conversely, Rutin has a measured impact on potentially pathogenic bacteria, such as certain strains of Escherichia coli. Through mechanisms that are still being unraveled, Rutin appears to act as a regulator, preventing the undue proliferation of harmful microbes that can contribute to intestinal inflammation and compromise the integrity of the gut barrier. Rutin’s modulation of microbial composition extends beyond bacteria to include other microbial inhabitants, such as fungi.35 Through its nuanced interactions, Rutin influences the fungal community in the gut. This dynamic modulation not only contributes to the overall resilience of the gut ecosystem but also hints at Rutin’s potential to maintain the delicate balance between microbial diversity and stability.36 Importantly, Rutin’s influence on microbial composition is not a one-size-fits-all scenario but rather a tailored response to the specific context of the gut environment. It adapts its impact based on the existing microbial milieu, promoting a balanced and harmonious ecosystem that aligns with the host’s health objectives.37
Effects on microbial metabolites
Within the complex realm of the gut microbiota, metabolic interactions form the harmonic undercurrents that profoundly influence host health. Rutin, as it travels through the gastrointestinal tract, becomes a key contributor to this metabolic symphony, impacting the production of microbial metabolites. One notable effect of Rutin is its promotion of SCFA production. SCFAs, including acetate, propionate, and butyrate, serve as vital metabolites with diverse roles in maintaining gut health. Rutin, acting as a facilitator in the fermentation process, encourages the growth of beneficial microbes that specialize in producing SCFAs. These microbial allies, such as Bifidobacteria and certain species of Firmicutes, transform dietary fibers into SCFAs, nourishing colonocytes and fortifying the integrity of the gut barrier.38
Among SCFAs, butyrate stands out as a key player, known for its anti-inflammatory properties and its role in promoting colonic health. Rutin’s impact on butyrate production, particularly through its influence on specific microbial communities, underscores its potential as a modulator of gut health. Beyond SCFAs, Rutin’s modulation extends to other microbial metabolites, including polyphenol-derived metabolites. The interaction between Rutin and gut microbes results in the transformation of its parent compound into diverse metabolites with unique bioactivities. These metabolites, often more bioavailable and bioactive than the original Rutin, contribute to the overall anti-inflammatory and antioxidant milieu within the gut.39
Moreover, Rutin’s influence on microbial metabolism extends to the production of secondary bile acids. Through interactions with specific microbial populations, Rutin shapes the bile acid profile within the gut. This modulation is of particular significance, as bile acids not only influence host metabolism but also exert regulatory effects on microbial communities.40 Rutin’s impact on bile acid metabolism thus represents a nuanced intervention in the intricate crosstalk between the host, the gut microbiota, and microbial metabolites. Importantly, Rutin’s effects on microbial metabolites are context-dependent, adapting to the prevailing gut environment. This adaptive modulation allows Rutin to contribute to the maintenance of a dynamic and resilient gut ecosystem, fostering an environment where beneficial metabolites flourish while potentially harmful ones are kept in check.41
Gut microbiota modulation by rutin
Within the intricate realm of the gut microbiota, metabolic interactions form the harmonic undercurrents that profoundly influence host health. As Rutin journeys through the gastrointestinal tract, it becomes a key contributor to this metabolic symphony, impacting the production of microbial metabolites. One notable effect of Rutin is its promotion of SCFA production. SCFAs, including acetate, propionate, and butyrate, are vital metabolites with diverse roles in maintaining gut health. Acting as a facilitator in the fermentation process, Rutin encourages the growth of beneficial microbes that specialize in producing SCFAs. These microbial allies, such as Bifidobacteria and certain species of Firmicutes, transform dietary fibers into SCFAs, contributing to the nourishment of colonocytes and fortifying the integrity of the gut barrier.42
Among SCFAs, butyrate stands out as a key player, known for its anti-inflammatory properties and its role in promoting colonic health. Rutin’s impact on butyrate production, particularly through its influence on specific microbial communities, underscores its potential as a modulator of gut health. Beyond SCFAs, Rutin’s modulation extends to other microbial metabolites, including polyphenol-derived metabolites. The interaction between Rutin and gut microbes results in the transformation of its parent compound into diverse metabolites with unique bioactivities. These metabolites, often more bioavailable and bioactive than the original Rutin, contribute to the overall anti-inflammatory and antioxidant milieu within the gut.43
Moreover, Rutin’s influence on microbial metabolism extends to the production of secondary bile acids. Through interactions with specific microbial populations, Rutin shapes the bile acid profile within the gut. This modulation is particularly significant, as bile acids not only influence host metabolism but also exert regulatory effects on microbial communities. Rutin’s impact on bile acid metabolism represents a nuanced intervention in the intricate crosstalk between the host, the gut microbiota, and microbial metabolites. Importantly, Rutin’s effects on microbial metabolites are context-dependent, adapting to the prevailing gut environment. This adaptive modulation allows Rutin to contribute to the maintenance of a dynamic and resilient gut ecosystem, fostering an environment where beneficial metabolites flourish while potentially harmful ones are kept in check.44
Rutin in colitis models
Animal studies
A study has examined the impact of rutin on chronic ulcerative colitis in rat models. It was observed that the administration of rutin resulted in a notable reduction in the generation of inflammatory mediators and a decrease in the extent of colonic damage. Rutin exhibited a regulatory effect on the expression of tight junction proteins, proteins responsible for mucus secretion, the proliferation and apoptosis of epithelial cells, and additionally facilitated the augmentation of regulatory T cells.33
A study investigated the anti-inflammatory and antioxidant effects of rutin on 2,4,6-trinitrobenzene sulfonic acid-induced ulcerative colitis in rats. The researchers found that rutin administration reduced the serum levels of nitric oxide, TNF-α, and IL-1 beta, as well as the colon tissue levels of lipid peroxidation. Rutin also increased the activities of antioxidant enzymes, reduced glutathione, and myeloperoxidase in the colon tissue.32
In an examination of the protective effects of a combination of rutin and ascorbic acid on experimental ulcerative colitis in rat models, it was observed that this combined treatment mitigated the histological and biochemical alterations induced by acetic acid in colon tissues. The combination of rutin and ascorbic acid also alleviated oxidative stress and inflammation by restoring the levels of malondialdehyde, glutathione, superoxide dismutase, catalase, nitric oxide, prostaglandin E2, TNF-α, and IL-10.45
Cell culture studies
A study investigated the anti-inflammatory and antioxidant effects of rutin on human intestinal epithelial cells (Caco-2) exposed to LPS. Pozo et al. found that rutin reduced LPS-induced production of pro-inflammatory cytokines, such as IL-6 and IL-8, and increased the expression of antioxidant enzymes, such as superoxide dismutase and CAT. Rutin also inhibited the activation of NF-κB and MAPK pathways in Caco-2 cells.46
In an investigation exploring the impact of rutin on human colon adenocarcinoma cells (SW480) treated with TNF-α, Lee and Seo revealed that rutin effectively inhibited TNF-α-induced apoptosis, preventing caspase-3 activation and DNA fragmentation in SW480 cells. Additionally, rutin suppressed the TNF-α-induced expression of COX-2, iNOS, and NF-κB, while concurrently boosting the expression of Nrf2 and HO-1 in SW480 cells, showcasing its anti-apoptotic and anti-inflammatory properties in this cellular context.47
Clinical evidence: Rutin in colitis patients
Human clinical trials
In a clinical trial conducted in China, the effects of a rutin-enriched extract derived from Sophora japonica L., a plant rich in rutin, were explored in patients with mild to moderate ulcerative colitis. The study included 60 participants who were randomly assigned to receive either the rutin-enriched extract (300 mg three times daily) or mesalazine (1.5 g three times daily) for a duration of eight weeks. Results indicated that the rutin-enriched extract exhibited efficacy comparable to that of mesalazine in improving clinical and endoscopic parameters associated with ulcerative colitis, including the Mayo score, rectal bleeding score, stool frequency score, and mucosal healing rate. Additionally, the rutin-enriched extract demonstrated the ability to lower serum levels of inflammatory markers such as TNF-α, IL-1 beta, and IL-8. Notably, the rutin-enriched extract was well-tolerated, with no serious adverse effects reported throughout the trial period.48
In an Indian clinical trial involving 50 participants with mild to moderate ulcerative colitis, the effects of a combination of rutin and ascorbic acid were investigated. The study employed a randomized design, with patients receiving either rutin (500 mg) and ascorbic acid (500 mg) twice daily or a placebo for a duration of 6 weeks. Results indicated a significant enhancement in clinical and endoscopic parameters associated with ulcerative colitis, including improvements in the Mayo score, rectal bleeding score, stool frequency score, and mucosal healing rate, for those administered the rutin and ascorbic acid combination. Furthermore, this combination resulted in a notable reduction in serum levels of markers related to oxidative stress and inflammation, such as malondialdehyde, nitric oxide, prostaglandin E2, TNF-α, and IL-10. Importantly, the rutin and ascorbic acid combination demonstrated good tolerability and did not elicit any serious adverse effects during the trial period.49
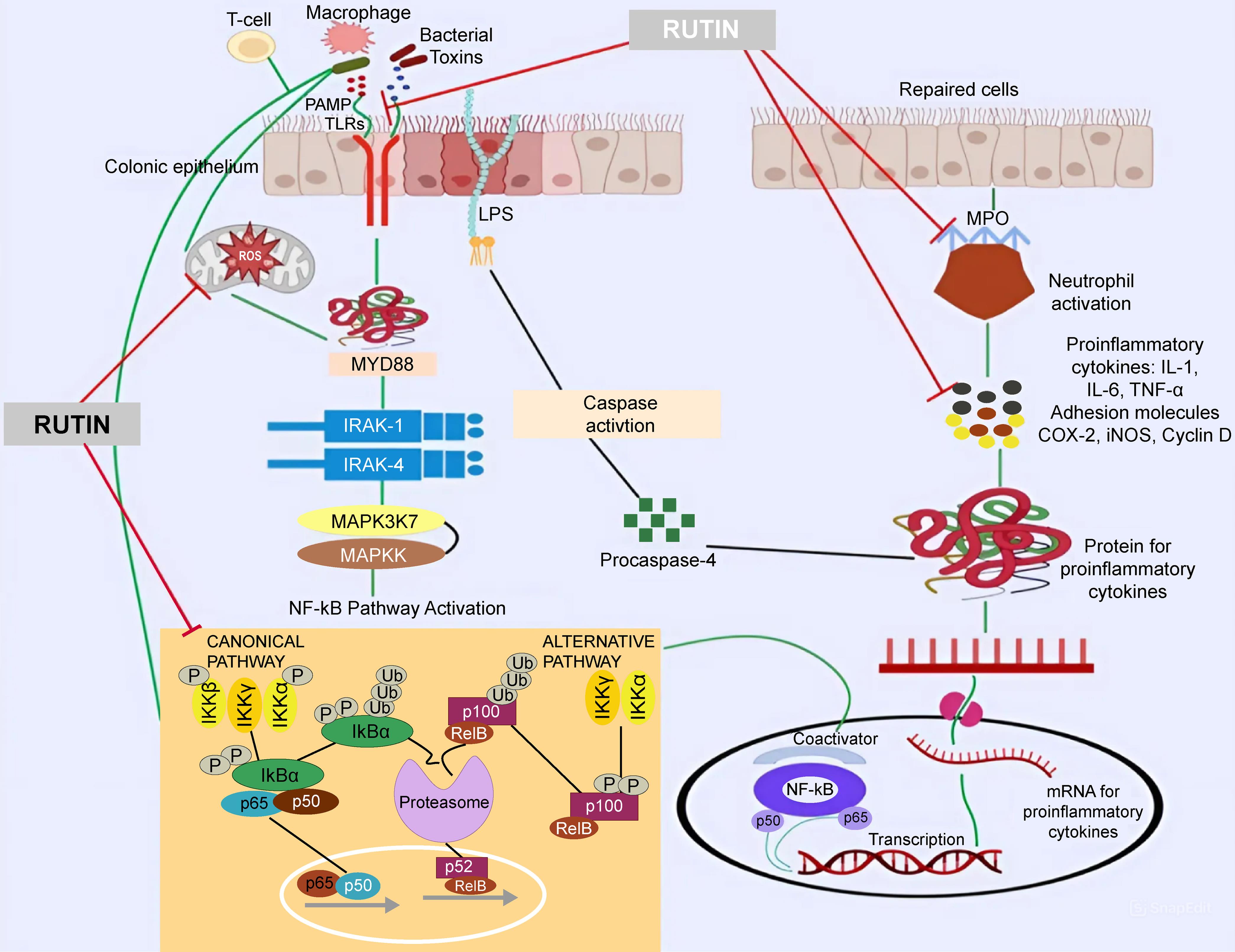
All studies are summarized schematically in Figure 3 and presented in Table 1. 32,33,45–49
COX, Cyclooxygenase; IL, Interleukin; LPS, Lipopolysaccharide; MPO, Myeloperoxidase;PAMP, Pathogen-Associated Molecular Pattern; TLRs, Toll-Like Receptors; TNF, Tumor Necrosis Factor.
Activity of rutin in ulcerative colitis, inflammation, and oxidative stress management
| S. No. | Study | Formulation | Animal/cell line used | Outcome | Ref. |
|---|---|---|---|---|---|
| 1. | Chronic ulcerative colitis | Isolated rutin compound | Chronic ulcerative colitis rat models | Results showed the reduction in the generation of inflammatory mediators and a decrease in the extent of colonic damage. | 33 |
| 2. | Anti-inflammatory and anti-oxidant | Rutin powder | Albino rats | Rutin also increased the antioxidant enzymes and reduced glutathione and myeloperoxidase activities in the colon tissue. Administration reduced the serum levels of nitric oxide, tumor necrosis factor-alpha, interleukin-1 beta, and the colon tissue level of lipid peroxidation. | 32 |
| 3. | Ulcerative colitis | Isolated Rutin and ascorbic acid | Ulcerative colitis in rat models | The study found that the rutin and ascorbic acid combination also alleviated oxidative stress and inflammation by reinstating the levels of malondialdehyde, glutathione, superoxide dismutase, catalase, nitric oxide, prostaglandin E2, tumor necrosis factor-alpha, and interleukin-10. | 45 |
| 4. | Anti-inflammatory and antioxidant | Isolated rutin | human intestinal epithelial cells (Caco-2) | It reduced the LPS-induced production of pro-inflammatory cytokines, such as IL-6 and IL-8, and increased the expression of antioxidant enzymes, such as SOD and CAT. Rutin also inhibited the activation of NF-κB and MAPK pathways in Caco-2 cells. | 46 |
| 5. | Anti-apoptotic, anti-inflammatory | Rutin powder | Human colon adenocarcinoma cells (SW480) | It was concluded that the rutin effectively inhibited TNF-α-induced apoptosis, preventing caspase-3 activation and DNA fragmentation in SW480 cells. Additionally, rutin suppressed the TNF-α-induced expression of COX-2, iNOS, and NF-κB, while concurrently boosting the expression of Nrf2 and HO-1 in SW480 cells, showcasing its anti-apoptotic and anti-inflammatory properties in this cellular context. | 47 |
| 6. | Ulcerative colitis | Rutin extract | 60 participants with mild to moderate ulcerative colitis | It was indicated that the rutin-enriched extract exhibited comparable efficacy to mesalazine in enhancing clinical and endoscopic parameters associated with ulcerative colitis, including the Mayo score, rectal bleeding score, stool frequency score, and mucosal healing rate. Also, it was evaluated that the capacity to lower serum levels of inflammatory markers such as tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8. | 48 |
| 7. | Ulcerative colitis | Rutin and ascorbic acid powder | 50 participants with mild to moderate ulcerative colitis | Evaluated results found significant enhancement in clinical and endoscopic parameters associated with ulcerative colitis, including improvements in the Mayo score, rectal bleeding score, stool frequency score, and mucosal healing rate, for those administered the rutin and ascorbic acid combination. Furthermore, the reduction in serum levels of markers related to oxidative stress and inflammation, such as malondialdehyde, nitric oxide, prostaglandin E2, tumor necrosis factor-alpha, and interleukin-10 was also observed. | 49 |
Challenges and future perspectives
Limitations of current research
While research on the therapeutic potential of rutin in colitis management has shown promising results, it is essential to acknowledge certain limitations that currently characterize this field:
Species variation
Much of the existing research relies on animal models, primarily rodents. Translating findings from these animals to human biology may be limited due to inherent differences in physiology and immune responses between species.
Diversity in colitis models
There is a variety of colitis models used in research, including chemically induced colitis, genetic models, and spontaneous colitis models. This diversity complicates the generalization of findings across different experimental setups, potentially limiting the broader applicability of the results.
Clinical relevance
The translation of findings from preclinical studies to clinical applications requires careful consideration. The limited number of clinical trials directly exploring the effects of rutin on human colitis presents a challenge in establishing its direct clinical relevance.
Mechanistic understanding
While research has shed light on the molecular mechanisms underlying rutin’s effects, a comprehensive understanding of its precise mode of action in the complex context of colitis is still evolving. Further elucidation of specific pathways and targets is needed.
Optimal dosage and administration
Determining the optimal dosage and administration protocols for rutin in the context of colitis remains an open question. Variability in dosage across studies may impact the consistency of results and hinder the establishment of standardized therapeutic regimens.
Long-term effects and safety
The long-term safety profile of rutin, especially in chronic conditions like colitis, requires more extensive investigation. Understanding potential side effects and ensuring the sustained efficacy of rutin over extended periods is crucial for its clinical applicability.
Human studies and clinical trials
The number of clinical trials involving rutin for colitis in human subjects is limited. Expanding human-based studies is essential to validate preclinical findings and understand the real-world effectiveness and safety of rutin in diverse populations.
Interactions with standard treatments
Many individuals with colitis are on standard pharmacological treatments. The potential interactions between rutin and these conventional treatments need careful consideration, and further research is required to determine compatibility and potential synergies or conflicts.
Limited mechanistic understanding of microbiota modulation
Although rutin’s impact on the gut microbiota is recognized, a more detailed understanding of the specific microbial strains influenced and the mechanisms underlying this modulation is crucial for a comprehensive grasp of its therapeutic effects.
Future research directions
Future investigations in the domain of rutin and its potential in managing colitis could follow several paths to enhance understanding and augment clinical applications. Here are four prospective avenues for research:
Expanded clinical trials
Conducting extensive clinical trials that encompass diverse participant cohorts is essential to substantiate the effectiveness and safety of rutin in colitis management. This would strengthen the evidentiary base and provide deeper insights into the applicability of outcomes across various patient groups.
Extended safety and impact assessments
Scrutinizing the long-term effects of rutin administration in colitis patients could unveil sustained efficacy patterns and safety considerations associated with prolonged use.
In-depth exploration of microbial modulation
Investigating the intricacies of rutin’s impact on gut microbiota will provide a detailed understanding of the affected microbial species and the involved signaling pathways, contributing to a more holistic understanding of rutin’s influence on the gut microbiome in colitis scenarios.
Synergistic therapies investigation
Exploring potential synergies from combining rutin with conventional therapeutic agents used in colitis management could enhance efficacy or enable reduced dosages of standard medications, opening avenues for optimized combination therapies.
These forthcoming research trajectories aimed to bridge existing knowledge gaps, refine perceptions of rutin’s therapeutic capacities in colitis, and establish a robust foundation for its integration into clinical practice. Systematic exploration of these pathways will contribute to the evolution of more potent and individualized interventions for individuals grappling with colitis.50
Conclusions
The exploration of rutin’s potential in managing colitis has unveiled a promising avenue for therapeutic interventions. Key findings from both preclinical investigations and clinical trials indicate that rutin, whether used alone or in conjunction with other compounds, demonstrates significant efficacy in improving clinical and endoscopic parameters associated with colitis. Its positive effects extend beyond symptom alleviation, encompassing the modulation of oxidative stress, inflammation, and the delicate balance of the gut microbiota.
These revelations have profound implications for the future landscape of colitis management. Rutin emerges as a compelling candidate for further study and potential clinical integration, offering a natural and well-tolerated alternative or complement to existing treatments. The observed enhancements in histological, biochemical, and immunological markers underscore its multifaceted potential in addressing the intricate pathogenesis of colitis.
As we navigate these promising developments, future research directions should prioritize expansive clinical trials to ensure a comprehensive understanding of rutin’s efficacy across diverse patient demographics. Investigating the long-term effects and safety profiles, exploring the intricate mechanisms of gut microbiota modulation, and assessing synergies with conventional therapies are crucial steps toward refining the clinical application of rutin in colitis management.
In conclusion, the journey into rutin’s therapeutic potential for colitis reveals not only a potential remedy but also a nuanced approach to addressing the intricate facets of this inflammatory disorder. The implications are expansive, signaling a future where the incorporation of rutin into colitis management strategies could provide holistic and personalized avenues for advancing patient well-being.
Declarations
Acknowledgement
The authors thank CV Raman Global University, ISF College of Pharmacy, and Chitkara University for providing the necessary facilities for the compilation of this manuscript. They also express gratitude to Ms. Maitrayee Ghosh for her support and advice on improving this article.
Funding
The authors declare that this study received no funding.
Conflict of interest
All authors declare no conflict of interest.
Authors’ contributions
Writing the original draft, reviewing and editing (DR), drawing (DM), writing the original draft (MG), editing (DA), conceptualization and supervision (NKR), methodology and supervision (AS).

 Author information
Author information