Introduction
Cancer continues to be a substantial and urgent public health issue worldwide. Annually, a significant number of new cancer cases are identified, and regrettably, a considerable number of deaths are attributed to cancer.1 The prevalence of cancer significantly impacts individuals, families, communities, and healthcare systems globally. The increasing prevalence of cancer can be attributed to various factors, including lifestyle choices, environmental influences, genetic predispositions, and demographic trends in the aging population. Additionally, the intricate nature of cancer, characterized by numerous distinct types and subtypes, frequently necessitates specialized and resource-intensive therapeutic interventions.2
Efforts to address cancer encompass various aspects, including advancements in treatment modalities such as immunotherapy, targeted therapies, and precision medicine, as well as initiatives focused on prevention, early detection, and public health.3 Education and awareness initiatives, tobacco control interventions, immunization against oncogenic viruses, and cancer screening programs are essential elements of the worldwide effort to combat cancer.4,5 In recent years, the field of cancer immunotherapy has experienced a surge in interest and research, especially in the development of novel nanoparticle-based delivery systems. Polymeric nanoparticles (PNPs) have emerged as promising candidates for targeted drug delivery and immunomodulation in cancer treatment due to their unique properties, such as high loading capacity, tunable size, and surface modification potential. In this review, we discuss the latest advancements in polymeric nanoparticle-based cancer immunotherapy, exploring their potential for overcoming the limitations of conventional cancer treatments and enhancing therapeutic efficacy. Additionally, we will discuss the challenges and future outlook of utilizing PNPs in cancer immunotherapy.
Cancer immunotherapy represents a groundbreaking shift in the field of cancer treatment, signaling the advent of a novel era defined by inventive methodologies. This therapeutic approach leverages the innate abilities of the immune system to identify and efficiently combat cancerous cells in the body. In contrast to traditional therapies that mainly focus on directly attacking cancer cells, immunotherapy utilizes complex mechanisms of the immune system to identify and eradicate abnormal cells.6 This innovative methodology involves the activation of immune cells to recognize cancer cells as nonself entities through the identification of distinct surface proteins or antigens that differentiate them from normal cells. Immunotherapy enhances the immune system’s ability to target and eliminate tumors by stimulating immune responses and disrupting inhibitory signals that cancer cells use to avoid detection.7 This individualized and focused methodology shows great potential, initiating a paradigm shift in which the innate immune system is mobilized as a powerful force in the battle against cancer.8
Cancer immunotherapy is a type of biological treatment that utilizes the body’s immune system to initiate an attack on tumor cells, leading to an antitumor effect.8 This methodology can instruct the immune system, allowing it to recognize and combat particular cancer cells while concurrently improving the efficacy of immune cells in eradicating cancerous tumors. It is crucial to emphasize that cancer immunotherapy not only effectively targets the primary tumor but also combats the metastasis of cancer to secondary locations by inducing a systemic immune response.9 In addition, it can inhibit the recurrence of tumors by inducing a cancer-specific memory immune response that remains vigilant and responsive when exposed to tumor-associated antigens.10
A variety of cancer immunotherapies, such as cancer vaccines, antibody therapy, cytokines, immune checkpoint inhibitors (ICIs), adoptive cell transfer (ACT), and oncolytic viruses, have been developed.11 Among these methodologies, the commercial triumph of ICIs and chimeric antigen receptor (CAR)-T cells has propelled the field of cancer immunotherapy into the limelight.12 Although immunotherapies have made significant advancements in cancer treatment by leveraging the innate immune response of the body, they still face certain obstacles. One of the major challenges is the limited capacity of these therapies to efficiently infiltrate tumors and achieve their desired outcomes.13 Inadequate tumor penetration can result in nonuniform dispersion of the treatment within the tumor volume, which may leave certain cancer cells unaffected and diminish the overall efficacy of the therapy. Furthermore, systemic toxicity is an additional concern.14 While immunotherapies are specifically engineered to selectively target cancer cells, there is potential for unintended activation of the immune system against normal, healthy tissues, resulting in adverse effects.15 It is imperative to overcome these challenges to maximize the efficacy of immunotherapies and enhance their wider utility in the treatment of diverse cancer types.8,16
The objective of targeted drug delivery is to accurately transport therapeutic agents to their designated site of action.16 Due to their versatile nature and effective interaction with diverse biological properties, nanoparticles provide a safe and effective method for delivering chemotherapeutic agents. Various types of nanocarriers have been investigated, including polymer-based, lipid-based, carbon-based, and metal-based nanoparticles.17 Among these, polymers are one of the most commonly utilized nanomaterials. Polymers designated for use in drug delivery systems must adhere to specific requirements, including biocompatibility, nontoxicity, and the absence of impurities.18,19 Furthermore, it is imperative that these entities exhibit prolonged circulation half-lives and possess a structurally adaptable framework capable of accommodating a diverse range of active molecules, such as small compounds, oligonucleotides, and peptides.20 The utilization of targeted PNPs provides an increased level of precision in regulating the pharmacokinetics, biodistribution, and bioavailability of drugs.21
PNPs offer a potential solution for overcoming the obstacles associated with current immunotherapies.22–23 These include the challenges of limited tumor penetration, systemic toxicity, immune evasion mechanisms employed by cancer cells, inadequate antigen presentation, and the immunosuppressive tumor microenvironment (TME). The utilization of nanoparticles presents a flexible and efficient framework for addressing the challenges associated with insufficient tumor penetration and the risk of systemic toxicity.24 By leveraging their distinctive characteristics, PNPs facilitate the targeted and accurate administration of immunomodulatory agents, specifically to the tumor site.25 By incorporating these therapeutic agents into PNPs, it is possible to control their release kinetics, thereby achieving a prolonged and targeted impact specifically within the tumor microenvironment. This focused methodology improves therapeutic efficacy while reducing the potential for systemic adverse reactions. Moreover, PNP systems can be engineered to traverse biological barriers and enhance intratumoral penetration, thereby mitigating the challenge of heterogeneous distribution.26 PNPs have the potential to significantly transform cancer immunotherapy by offering a customized and effective approach to improve treatment results while addressing the obstacles that have impeded the complete realization of immunotherapeutic capabilities.22
Compared with other nanotechnologies, PNPs have distinct advantages in terms of targeting ability. Unlike liposomes, which have limited stability and susceptibility to premature drug release, PNPs offer enhanced stability and controlled release properties, ensuring targeted delivery of therapeutic payloads. Moreover, compared with metallic nanoparticles, PNPs exhibit superior biocompatibility and tunable surface properties, minimizing potential cytotoxicity and immune responses. Compared to dendrimers, which often face challenges related to synthesis scalability and toxicity concerns, PNPs offer a more scalable and versatile platform for targeted drug delivery.27
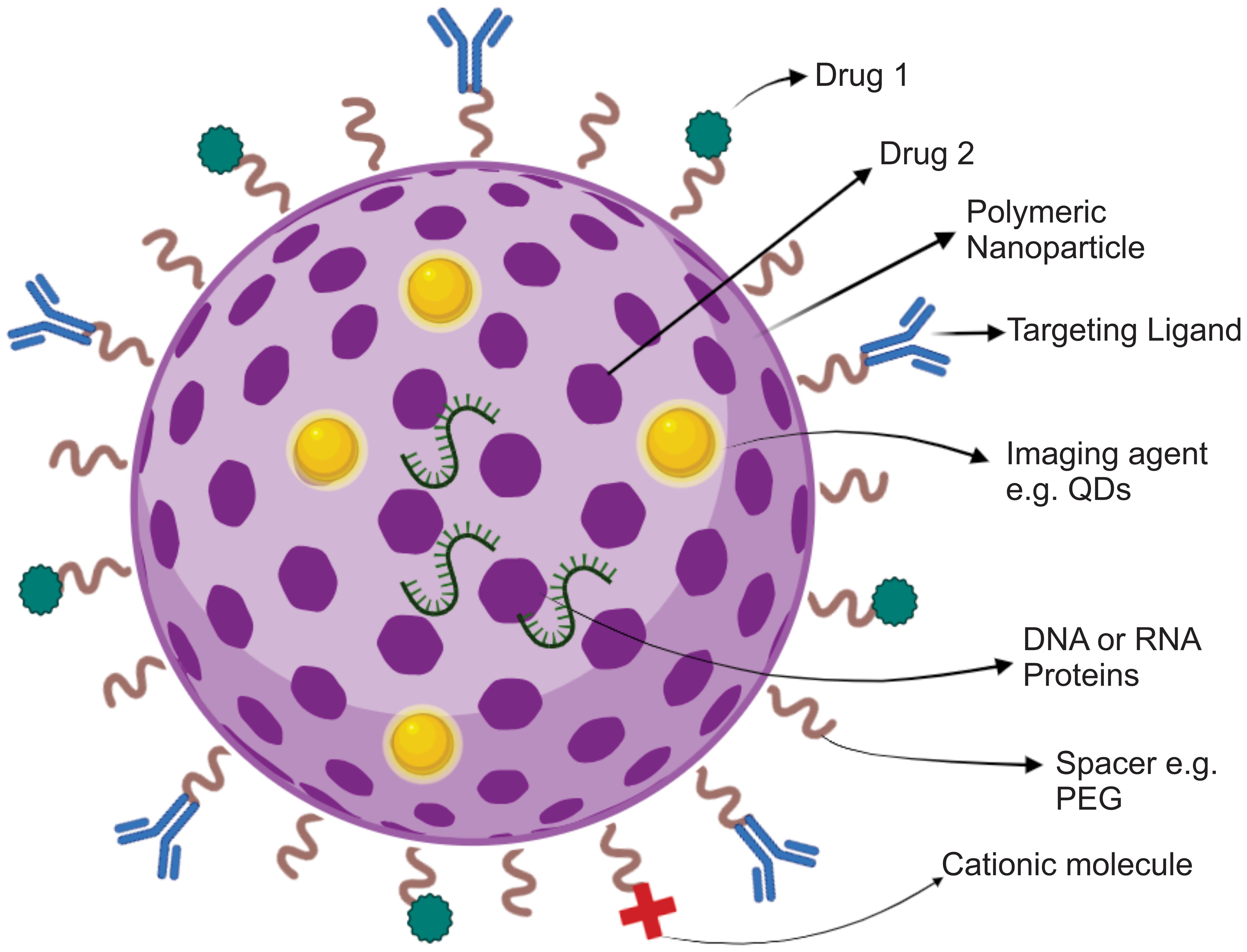
PNPs have undergone significant advancements, becoming adaptable and highly customizable platforms for efficient drug delivery. This has led to their potential to significantly transform the field of medical treatments. Surface engineering is a process that modifies the external layer of PNPs to improve their functionality in drug delivery and therapeutic uses.27,28 A frequently employed method involves the application of hydrophilic polymers, such as polyethylene glycol (PEG), to coat PNPs. This technique is commonly referred to as PEGylation.29 This phenomenon is known as the “stealth effect”, and it enhances the stability and prolongs the circulation of the substance in the bloodstream.30,31 Moreover, surface modification facilitates the conjugation of ligands, antibodies, peptides, or small molecules, allowing for the specific targeting of particular cells or tissues, thereby enhancing the accuracy and effectiveness of drug administration. Additionally, the incorporation of targeting agents within the PNP structure results in improved controlled release and targeted therapeutic efficacy.32 This review aims to provide a comprehensive and critical analysis of the preparation methods and applications of polymer nanoparticles for cancer immunotherapy. By evaluating the current state of research in this field, the significance of polymer nanoparticles in enhancing the delivery and efficacy of immunotherapeutic agents will be highlighted. Additionally, the review seeks to identify the challenges and opportunities associated with the development and utilization of polymer nanoparticles for cancer immunotherapy, offering insights for future research directions and clinical applications.
Design and preparation of polymeric nanoparticles
The synthesis of PNPs encompasses a range of techniques that can be customized according to the characteristics of the drug to be encapsulated and the specific demands of its delivery route.33 Typically, two main approaches are utilized: the dispersion of preformed polymers and the polymerization of monomers.
The dispersion of preformed polymers involves utilizing preexisting polymers as the initial substance. These polymers often possess biocompatibility and biodegradability properties. They may already exist in various physical forms, such as microparticles, nanoparticles, or macrostructures. Nanoparticles are commonly dispersed within a liquid medium, which can be either an organic solvent or water. Subsequently, a series of processes are employed to induce their fragmentation into smaller nanoparticles. Methods such as nanoprecipitation, emulsification, and solvent evaporation are frequently employed.34 Pharmaceutical compounds are commonly incorporated into nanoparticle synthesis procedures, either during or after the formation stage, resulting in their encapsulation within the polymer matrix.35
In polymerization of monomers, nanoparticles are synthesized through a bottom-up approach. Monomers, which serve as the fundamental units of polymers, are selected according to the intended characteristics of the resultant nanoparticles. The monomers undergo a polymerization process to generate a polymer chain. This phenomenon can occur through different mechanisms, such as emulsion polymerization, mini-emulsion polymerization, or precipitation polymerization.36 During this procedure, the drug can be integrated directly into the evolving polymer framework, resulting in the formation of drug-containing nanoparticles. The following techniques are employed for the preparation of PNPs:
Solvent evaporation method
Solvent evaporation is a conventional technique employed for the fabrication of PNPs using preexisting polymers. The process begins with the formation of an oil-in-water emulsion, resulting in the production of nanospheres. The polymer is dissolved in an organic phase, and the drug is integrated into the solution either through dissolution or dispersion. In the past, dichloromethane and chloroform were commonly utilized, but ethyl acetate, which is considered safer for biomedical applications, is now the preferred choice.37 Concurrently, a solution is prepared, consisting of an aqueous phase that contains a surfactant, such as polyvinyl acetate. High-speed homogenization or ultrasonication is utilized to create nanodroplets by emulsifying the organic solution within the aqueous phase.38 During polymer solvent evaporation process, the nanoparticles become suspended, enabling the solvent to exit the system. Evaporation can be induced through agitation at ambient temperature or by gradually reducing the pressure. Once the solvent has completely evaporated, the nanoparticles solidify and gather. These nanoparticles are then subjected to centrifugation for washing and are finally freeze-dried to facilitate storage. This series of steps effectively results in the formation of nanospheres.39
Emulsification/solvent diffusion method
The emulsification process involves the dispersion of one liquid that is not capable of mixing with another liquid. The diffusion process entails the creation of an emulsion in which oil is dispersed in water. This is achieved by mixing a solvent that is partially soluble in water, a polymer, and a drug with an aqueous solution containing surfactants. The internal phase is composed of a partially water-miscible organic solvent, such as benzyl alcohol or ethyl acetate, which has undergone presaturation with water to attain thermodynamic equilibrium.40 The dilution process involves the addition of water, which causes the solvent to diffuse from droplets into the surrounding medium. This diffusion leads to the creation of colloidal particles. In the realm of nanospheres, the incorporation of a minute amount of oil into the organic phase can enhance the generation of nanocapsules. The selection of a solvent elimination technique is based on its boiling point, which can be achieved through either evaporation or filtration methods. This method generates nanoparticles with a size distribution ranging from 80 to 900 nm. This technique is commonly utilized, but it requires substantial partitioning of the aqueous phase and can lead to the potential diffusion of hydrophilic drugs into the aqueous phase.41,42
Emulsification/reverse salting-out method
Emulsification/reverse salting-out is a modified version of the emulsification/solvent diffusion method, characterized by a distinct composition of the oil-in-water emulsion. The process employs a water-miscible polymer solvent such as acetone or ethanol. The aqueous phase consists of a gel matrix, a salting-out agent (either electrolytes such as MgCl2 or nonelectrolytes such as sucrose), and a colloidal stabilizer. By reaching the point of saturation in the aqueous phase, the ability of acetone and water to mix decreases, allowing for the creation of an emulsion. The obtained emulsion is subsequently diluted to promote the diffusion of organic solvents, the precipitation of polymers, and the formation of nanospheres. Cross-flow filtration is employed to eliminate the remaining solvent and salting-out agents.43,44
Nanoprecipitation method
Nanoprecipitation, also referred to as the solvent displacement method, utilizes two solvents that are capable of mixing. The process begins by dissolving a polymer into a solvent that is compatible and capable of forming a homogeneous mixture, such as acetone or acetonitrile. These non-water-soluble solvents can be easily removed by evaporation. The method is based on the deposition of the polymer at the interface as the organic solvent transitions from a lipophilic solution to an aqueous phase. The polymer is gradually introduced into the aqueous solution, either by adding small drops or by controlling the rate of addition while stirring. The solvent used is capable of mixing with water. The rapid diffusion phenomenon leads to the instantaneous formation of nanoparticles, as they tend to evade water molecules. As the solvent is depleted from the nanodroplets, the polymer precipitates, resulting in the formation of nanocapsules or nanospheres.45 In general, the organic phase is introduced into the aqueous phase, although the process can be reversed without affecting nanoparticle formation. While surfactants can be utilized to enhance the stability of colloidal suspensions, they are not essential for nanoparticle formation. Nanoprecipitation produces nanoparticles that have precise characteristics and a limited range of sizes. These nanoparticles are typically smaller than those obtained through emulsification solvent evaporation. This technique is frequently employed for the synthesis of PNPs with an average diameter of approximately 170 nm, as well as nanospheres and nanocapsules. Nanospheres are generated through the dissolution or dispersion of the active component within the polymer solution. On the other hand, nanocapsules are produced by dissolving the drug in oil, which is subsequently emulsified in the organic polymeric solution before being dispersed into the external emulsion phase.39
Immunomodulation and immune activation
In addition to serving as carriers for therapeutic agents, PNPs possess inherent immunomodulatory properties that can effectively modulate the tumor microenvironment. Surface modification of PNPs with immune-stimulating ligands or antigens can promote dendritic cell activation and antigen presentation, thereby eliciting robust antitumor immune responses. Furthermore, the sustained release of immunomodulatory agents from PNPs can contribute to the activation of cytotoxic T cells and natural killer cells, bolstering the immune response against cancer.
Loading and delivery of immune modulators
The encapsulation of immune modulators within PNPs is a pivotal stage in the advancement of targeted immunotherapy approaches. Immune modulators refer to substances that can stimulate, inhibit, or regulate the immune system’s response to various diseases, such as cancer.46 These substances may include cytokines such as interleukins and interferons, checkpoint inhibitors like anti-PD-1 antibodies, Toll-like receptor agonists, or other agents with immunomodulatory properties.47,48 The utilization of PNPs has various benefits, such as controlled release, enhanced stability, and precise delivery of these modulators. The subsequent methods outline the loading techniques employed for loading immunomodulators into PNPs:
Simple mixing
In some cases, the immune modulator may be included directly in the polymer solution during the production of PNPs. This method is suitable for hydrophilic immune modulators that demonstrate solubility or dispersibility inside the polymer solution.
Double emulsion method
To address the issue of hydrophobic immune modulators or those with low solubility in the polymer solution, the double emulsion (water-in-oil-in-water, W/O/W) method can be employed. During this process, the immune modulator is dissolved in an organic solvent, and then the resulting organic phase is emulsified with an aqueous polymer solution. This approach allows the integration of the immune modulator into the PNP core.
Coprecipitation
The coprecipitation process involves dissolving both the polymer and the immune modulator in a shared solvent, which is subsequently precipitated by the addition of a nonsolvent. This phenomenon can result in the generation of nanoparticles wherein the immune modulator is evenly dispersed within the polymer matrix.
PNPs provide a flexible framework for the loading and controlled release of immunomodulators.49 This enhances the precision of immune responses and mitigates the potential for systemic toxicity. These strategies exhibit significant potential in enhancing cancer immunotherapy and other immune-related treatments by optimizing therapeutic outcomes while minimizing adverse effects on patients’ overall well-being. The targeted administration of immunomodulators using PNPs mitigates the risk of systemic toxicity. Given the potent impact immunomodulators can have on the entire immune system, it is crucial to minimize their exposure to healthy tissues to ensure patient safety. PNPs can accomplish this by selectively delivering immunomodulators to the tumor site or the tumor microenvironment.50
Methodologies for targeted delivery of immunomodulators
Encapsulation of immunomodulators
Immunomodulatory substances, such as cytokines or checkpoint inhibitors, can be enclosed within the polymeric matrix of PNPs.51 The process of encapsulation serves to safeguard immunomodulators from degradation within the bloodstream while also enabling the regulated and gradual release of substances.48 The selection of a polymer and its specific characteristics, such as hydrophobicity and biodegradability, can impact the rate at which a substance is released. Hydrophobic polymers such as poly(lactic-co-glycolic acid) (PLGA) are frequently employed to achieve sustained release of immunomodulators.47,52
Surface modification with ligands
PNPs can undergo surface functionalization through the attachment of ligands. These ligands are designed to specifically target immune cells or tissues. In this case, it is possible to conjugate antibodies or peptides that exhibit affinity toward receptors present on immune cells to the surface of the PNP.53,54 This targeting strategy employs a mechanism to specifically deliver immunomodulators to the desired immune cells, thereby augmenting the specificity of the immune response.55,56 By selectively modulating immune cells within the localized environment of the tumor, PNPs can effectively counteract the immunosuppressive characteristics exhibited by the tumor (Fig. 1).
Stimulus-responsive PNPs
Intelligent programmable nanoparticle systems can be designed to detect and react to precise environmental stimuli present in the human body. In the context of immunomodulation, the pH of the tumor microenvironment plays a pivotal role as a significant indicator. Numerous tumors exhibit a marginally lower pH than regular tissues.57 PNPs can be engineered to selectively release immunomodulators in response to the pH gradient, thereby confining the therapeutic effects to the tumor site. This approach mitigates overall exposure to the system and decreases the likelihood of unintended impacts on nontarget elements.58
Codelivery with antigen or vaccines
PNPs can coadminister immunomodulatory agents with tumor antigens or vaccinations in a concurrent manner. This methodology enhances the efficiency of the immune response by simultaneously activating the immune system via the introduction of antigens and modulating its activity through the use of immunomodulators. The simultaneous release of both components from PNPs may enhance the efficiency of the immune response.59,60
Dendritic cell targeting
Dendritic cells are pivotal in the initiation and regulation of immune responses. PNPs can be engineered with a high degree of specificity to selectively bind to dendritic cells.61 This enables the direct delivery of immunomodulators to these specialized antigen-presenting cells (APCs). This methodology facilitates the presentation of antigens and the activation of the immune system, specifically at the location of the tumor, while reducing overall exposure to the rest of the body.62
Antigen delivery and presentation
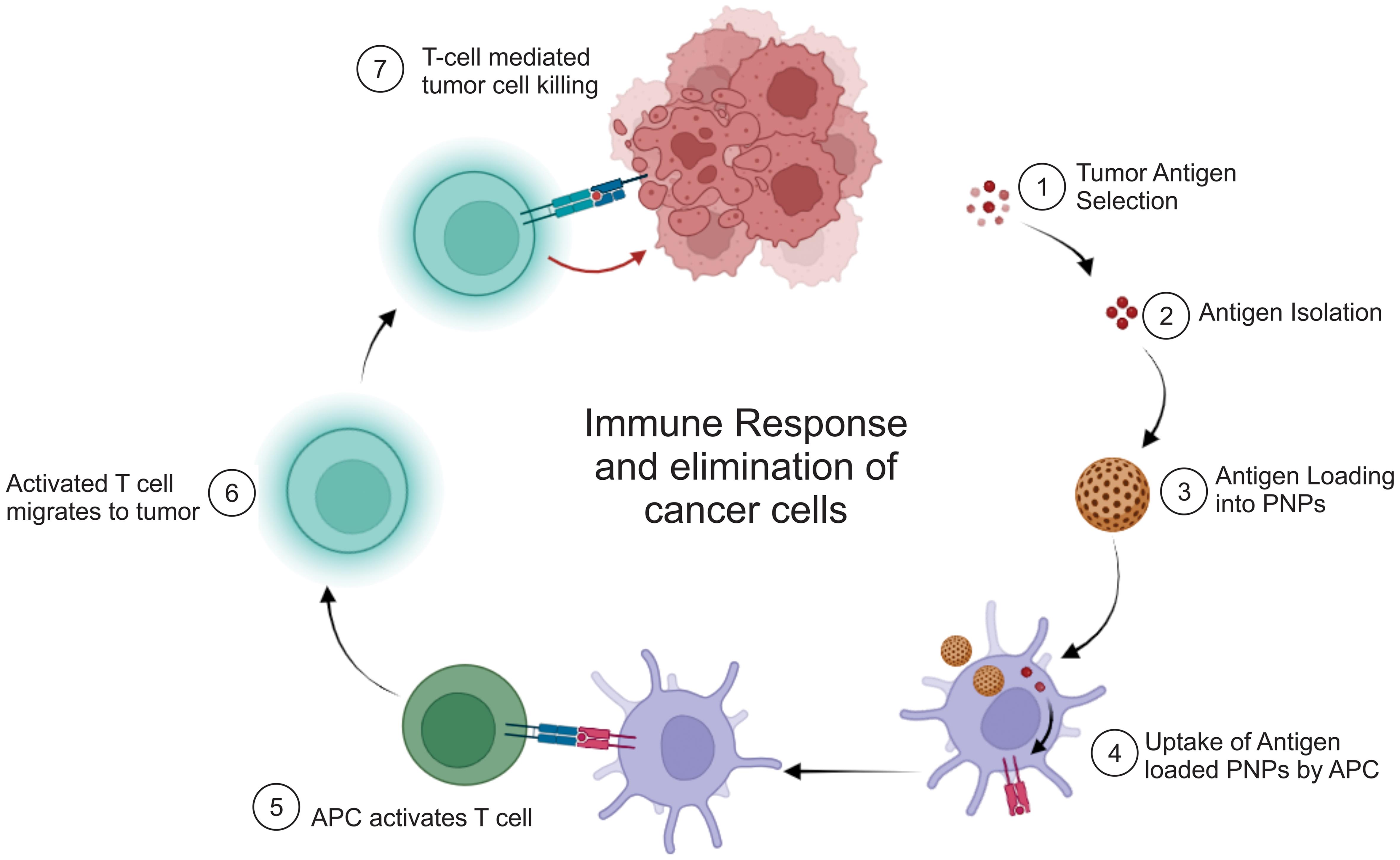
PNPs provide a highly effective approach for delivering tumor-associated antigens (TAAs) and immunomodulatory molecules to APCs.63 This process promotes the activation, expansion, and response of T cells, as illustrated in Figure 2. On the other hand, the effective transport of TAAs and tumor lysates in soluble form is hindered by their rapid elimination from the body and inadequate absorption by APCs. Surface conjugation or encapsulation of entire protein or peptide antigens onto nanoparticles has been found to be extremely advantageous in this context, as these methodologies improve the process of capturing antigens, cross-presentation, and the development of APCs.64
Mechanistic of immuno-activation by polymeric nanoparticles
The mechanism of immune activation by PNPs in cancer immunotherapy is multifaceted and involves several interconnected steps aimed at enhancing the body’s immune response against tumor cells.65,66
Immunotherapeutic modalities within the domain of cancer immunotherapy have been recognized as efficacious. This phenomenon may be attributed largely to their distinctive capacity to encapsulate tumor antigens and facilitate their uptake by APCs.67 This process is a crucial step in harnessing the immune system’s ability to detect and combat cancer. The first phase in this process is the identification and subsequent selection of antigens that exhibit specificity toward the tumor or are closely linked to it. These antigens are often composed of proteins or peptides that exhibit specific expression patterns in malignant cells but are absent in normal cells. Molecular markers serve as entities that may be recognized by the immune system as foreign or deviant.
Tumor antigens can be acquired from neoplastic cells using diverse methodologies.68 These antigens can exist as complete proteins or as distinct peptide fragments that originate from proteins associated with cancer. Furthermore, tumor-associated antigens can be artificially produced or replicated through the use of recombinant DNA technology. After the acquisition of tumor antigens, they are inserted into PNPs. The loading process is commonly accomplished using techniques such as simple mixing or the double emulsion method. In the double emulsion technique, tumor antigens can be solubilized or dispersed within an aqueous phase. This aqueous phase is subsequently emulsified within an organic polymer solution to generate PNPs. This procedure involves the encapsulation of antigens within the PNPs.69
Following the loading of tumor antigens, PNPs are administered to patients, usually through injection. PNPs are introduced into the circulatory system and subsequently migrate to the tumor site. At the tumor site, APCs, specifically dendritic cells, are found. Dendritic cells possess a unique ability to capture and present antigens to T cells, thereby initiating an immune response. Upon the arrival of tumor antigen-loaded PNPs at the tumor site, dendritic cells phagocytose these PNPs, efficiently internalizing the encapsulated antigens. After internalization by dendritic cells, the PNPs undergo processing. The tumor antigens enclosed in a protective layer are discharged within the dendritic cells, where they undergo degradation into smaller peptide fragments. These fragments are subsequently loaded onto molecules known as major histocompatibility complexes.70 Dendritic cells, which have now acquired antigen-major histocompatibility complexes on their surfaces, migrate toward secondary lymphoid organs such as lymph nodes.71 In this context, tumor antigens are presented to T cells, specifically CD8+ cytotoxic T cells. T cells possess receptors capable of identifying the presented antigens.72
Upon encountering dendritic cells that present tumor antigens, CD8+ T cells undergo activation. The activated cytotoxic T cells are now in a state of readiness to selectively identify and eliminate cancer cells that exhibit identical antigens. The process of recognizing and activating specific immune responses is crucial for initiating an efficient immune reaction against the tumor. Activated cytotoxic T lymphocytes (CTLs) can infiltrate the tumor microenvironment, identify cancer cells that express matching antigens, and initiate their eradication.72,73 The activation of the immune system can result in the eradication of malignant cells and facilitate the regression of tumors (Fig. 2).
Overcoming immune evasion and tumor microenvironment modulation
PNPs are a highly adaptable tool in the field of cancer immunotherapy. They can be modified through engineering techniques to specifically target and counteract the immunosuppressive tactics employed by cancer cells. Additionally, PNPs have the ability to modify unfavorable conditions in the tumor microenvironment, making them more conducive to effective treatment. By delivering checkpoint inhibitors, immune stimulants, and TME-remodeling agents directly to the tumor site, PNPs facilitate the infiltration of immune cells, stimulate immune responses against the tumor, and mitigate potential toxicity to the entire system. This approach represents a potential strategy for addressing immune evasion in cancer and enhancing the effectiveness of immunotherapies.74 The subsequent strategies employed by cancer cells to evade the immune system and alter the tumor microenvironment in the context of cancer immunotherapy are as follows:
Immune checkpoint inhibitors and immune stimulants
Cancer cells can manipulate immune checkpoints to avoid detection by the immune system. Checkpoint proteins, such as PD-1 and CTLA-4, located on immune cells, can bind to specific ligands on cancer cells. This interaction triggers the transmission of inhibitory signals that effectively reduce the strength of immune responses. This interaction establishes an immunosuppressive milieu that protects cancer cells against immune attack. PNPs have the potential to be modified to contain checkpoint inhibitors, such as anti-PD-1 or anti-CTLA-4 antibodies, as well as immune stimulants such as Toll-like receptor agonists.75 By deploying controlled release mechanisms at the tumor site, PNPs can reverse immune evasion, protecting immunotherapeutic molecules from degradation in the bloodstream. Additionally, PNPs facilitate the targeted accumulation of these molecules specifically within the tumor. Upon administration, checkpoint inhibitors block the interaction between checkpoint proteins on immune cells and their corresponding ligands on cancer cells. This mechanism inhibits inhibitory signals and restores the activity of cytotoxic T cells, enabling them to identify and eliminate cancerous cells. Immune stimulants can augment the functionality of immune cells, thereby amplifying the collective antitumor immune response.72
Remodeling the tumor microenvironment (TME)
The tumor microenvironment is frequently characterized by immunosuppression, which poses challenges for immune cell infiltration and cancer cell eradication. Immunosuppressive elements such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and immunosuppressive cytokines contribute to the establishment of an unfavorable milieu for immune responses.76 PNPs can be manipulated to modify the tumor microenvironment. They can administer therapeutic agents that specifically target and deplete immunosuppressive cells such as Tregs and MDSCs. Moreover, PNPs can transport pharmaceutical substances that can counteract the effects of immunosuppressive cytokines or enhance the mobilization of immune effector cells.77 By modulating the immunosuppressive microenvironment, PNPs facilitate the recruitment and activation of immune cells, including cytotoxic T cells and natural killer cells. These immune cells play a vital role in the recognition and eradication of cancer cells within the tumor.73,78
Combination therapies
PNPs enable the concurrent administration of multiple immunomodulatory agents. This facilitates the advancement of combination therapies, wherein checkpoint inhibitors, immune stimulants, and TME-remodeling agents collaborate synergistically to augment the antitumor immune response.79 PNPs, or polymeric nanoparticles, facilitate the controlled release of immunomodulators, which are substances that modify the immune response. This controlled release mechanism ensures the sustained and prolonged presence of immunomodulators, specifically at the tumor site, optimizing the therapeutic impact while minimizing the potential for systemic toxicity. The targeted administration of immunomodulators at the tumor site reduces contact with noncancerous tissues, thereby decreasing the likelihood of systemic side effects linked to these powerful substances.76
Clinical translation
In the past few decades, there has been notable progress in comprehending the regulatory mechanisms of antitumor immunity, specifically in relation to immune checkpoint pathways. These established pathways have facilitated the advancement of ICIs, which have significantly transformed the field of cancer therapy. Nevertheless, it is crucial to acknowledge that ICIs used as a standalone treatment may not produce favorable outcomes for every cancer patient.80
To overcome this clinical challenge, researchers have investigated the integration of PNP technology with immunotherapeutic agents or conventional cancer treatments, specifically those involving ICIs. One strategy entails leveraging traditional cancer therapies, including chemotherapy, photodynamic therapy, and radiotherapy, to activate the immune system’s targeted antitumor response.81 These therapeutic interventions induce immunogenic cell death in neoplastic cells, leading to the release of damage-associated molecular patterns that trigger the activation of APCs. Activated APCs phagocytose apoptotic tumor cells and subsequently present tumor antigens, thereby initiating T-cell responses.81,82
PNPs can transport chemotherapeutic agents to the tumor site, thereby augmenting the targeted antitumor immune response elicited by these agents. Through encapsulation, nanoparticles provide a protective barrier for chemotherapeutic drugs, shielding healthy tissues from the harmful effects of cytotoxicity. Additionally, this encapsulation technique improves the targeted delivery of drugs specifically to the tumor site. PNPs can be utilized for targeted delivery of ICIs, specifically to the tumor microenvironment. This facilitates the localized suppression of immune checkpoint pathways, such as PD-1/PD-L1 or CTLA-4, leading to the stimulation of T cells and a stronger immune response against tumors.82 PNPs enable accurate localization of specific cells or tissues, thereby facilitating the targeted delivery of immunotherapeutic agents to the desired location. This mechanism decreases the occurrence of unintended cell death, thereby improving the therapeutic ratio, particularly when used in conjunction with other treatments.83
In recent years, significant advancements have been made in cancer immunotherapy, leading to the approval of certain treatments by regulatory agencies such as the FDA. FDA approval was granted for the use of atezolizumab in conjunction with albumin-bound paclitaxel NP (nab-paclitaxel) for the treatment of advanced triple-negative breast cancer. Hensify®/NBTXR3, a medical product containing 50 nm crystalline hafnium oxide nanoparticles, has received regulatory approval in the European market for the treatment of locally advanced soft tissue sarcoma when used in conjunction with radiation therapy.84 The IMpower130 trial and other clinical trials have provided evidence of the effectiveness of combination treatments in prolonging overall survival and progression-free survival in individuals diagnosed with non-small cell lung cancer. Moreover, scientists have conducted investigations on RNA-formulated nanoparticles in clinical trials, evaluating their efficacy as independent therapies and in conjunction with immune checkpoint inhibitors. The objective of these trials is to leverage the capabilities of mRNA nanovaccines and other RNA-based therapies for cancer immunotherapy.85
In a breast cancer model involving macrophages, pH-responsive star-PLGA nanoparticles improved drug release and penetration.86 In a study of brain metastatic breast cancer cells, the small size and anionic surface of polymeric particles made them more permeable to the brain compared to PEGylated nanoparticles alone.87 Therapeutic cancer vaccines, which are designed to amplify tumor-specific T-cell responses, are among the most powerful tools for effective cancer immunotherapy.88 One study showed that PNPs conjugated with an anti-EGFR monoclonal antibody and 5-fluorouracil (5-FU) had increased cellular uptake and increased cytotoxicity, thereby providing a good therapeutic effect on EGFR-positive colorectal cancer.89
Future perspectives and challenges
The potential of PNPs in cancer immunotherapy is promising, but several challenges and opportunities need to be addressed in the future. The ongoing advancement of patient-specific therapeutics using PNPs has significant potential. The progress in genetics and the discovery of biomarkers has the potential to provide polymeric nanoparticulate therapies that are specifically customized to individual patients, hence maximizing the therapeutic efficacy. The investigation of combination therapy remains a major area of focus. The potential for synergistic anticancer effects may be achieved by combining PNPs with other immunotherapeutic methods, such as chimeric antigen receptor T-cell treatment or oncolytic viruses. The exploration of this field has the potential to unveil novel pathways for improved cancer therapy. Furthermore, it is important to consider the scalability and cost-effectiveness of PNP manufacturing to facilitate its wider use in clinical settings. Ensuring the widest accessibility of these therapies require streamlined production procedures and affordability.
Future trends in PNP design for cancer immunotherapy are poised to revolutionize treatment approaches. One emerging trend is the integration of machine learning algorithms into the design process, enabling the rapid and systematic optimization of nanoparticle properties for enhanced therapeutic efficacy. Machine learning algorithms can analyze vast datasets of nanoparticle characteristics, including size, shape, surface chemistry, and payload composition, to identify optimal formulations that maximize drug delivery, immune activation, and tumor targeting. This approach holds promise for accelerating the development of next-generation nanoparticle-based immunotherapies tailored to individual patient profiles. Furthermore, the exploration of novel materials for nanoparticle fabrication is another promising avenue. Advances in nanomaterial science offer opportunities to engineer nanoparticles with enhanced biocompatibility, stability, and specificity for targeting immune cells and tumor microenvironments. By leveraging innovative materials such as biomimetic nanoparticles, responsive polymers, and self-assembling nanomaterials, researchers can overcome existing limitations and unlock new capabilities in cancer immunotherapy.
Thorough study is required to assess the long-term safety and possible immunotoxicity of PNPs. Gaining a comprehensive understanding of the long-term interactions between nanoparticles and the immune system is crucial for safeguarding the overall health and welfare of patients. Moreover, it is essential to address the issue of tumor heterogeneity. Developing targeted therapeutic strategies to effectively address the heterogeneous cell populations within tumors, particularly those resistant to conventional therapies, remains a significant challenge. The attainment of regulatory clearances and successful clinical translation will be of utmost importance. To facilitate the transition from preclinical efficacy to clinical implementation, it is essential to establish rigorous clinical studies, obtain regulatory support, and foster cooperation with industry stakeholders. The successful resolution of these problems will be of utmost importance for effectively using all the capabilities of PNPs in the continuous battle against cancer.
Conclusions
PNPs are an advanced and highly promising platform for cancer immunotherapy. The precise delivery of immunomodulatory agents, targeting of specific tumor antigens, and reshaping of the tumor microenvironment provide substantial benefits in the battle against cancer. PNPs have exhibited significant promise in preclinical investigations and ongoing clinical trials, showing their ability to augment the specificity and effectiveness of cancer immunotherapies. From the administration of immune checkpoint inhibitors to the facilitation of antigen presentation, these nanoparticles have demonstrated adaptability and efficacy in multiple facets of cancer therapy.
Nevertheless, various obstacles need to be addressed in the field of PNPs, including issues related to manufacturing scalability, long-term safety, and regulatory hurdles. It is imperative to overcome these challenges to fully harness the capabilities of PNPs in clinical practice. Notwithstanding these obstacles, the outlook for the use of PNPs in cancer immunotherapy appears encouraging. Through ongoing research, technological advancements, and effective cooperation among scientists, clinicians, and regulatory agencies, PNPs are assumed to play a crucial role in the progression of cancer treatment. This will lead to improved patient outcomes and significantly contribute to combating this formidable disease.
Declarations
Acknowledgement
The authors wish to express their gratitude to experts from the Lloyd Institute of Management and Technology, Greater Noida, for providing all the essential facilities and advice for conducting the review.
Funding
Not applicable.
Conflict of interest
The authors declare that they have no conflicts of interest.
Authors’ contributions
Conceptualization, Writing-original draft and editing, Correspondence (AKP), Supervision (VA). Both authors read and approved the submitted version.

 Author information
Author information