Introduction
Sepsis represents a clinical syndrome triggered by a dysregulated inflammatory response to infection, resulting in physiological and organ dysfunctions,1,2 often leading to multi-organ failure. Acute kidney injury (AKI) is a critical health concern due to its high incidence, multifactorial etiology, and significant impact on morbidity and mortality, warranting focused research and clinical management efforts worldwide. AKI is the most common complication of sepsis, occurring in over 50% of cases.1 Among these, 15–20% of patients with Sepsis-Associated AKI (SA-AKI) require renal replacement therapy (RRT).2,3 Beyond its association with short-term mortality, SA-AKI also contributes to the development and progression of chronic kidney disease (CKD), end-stage renal disease, and increased long-term mortality.2,4 Management of SA-AKI primarily includes early fluid resuscitation, timely administration of appropriate antibiotics, vasopressors, infection source control, and supportive treatment for renal dysfunction.5 The Kidney Disease: Improving Global Outcomes (hereinafter referred to as KDIGO) AKI guidelines recommend continuous RRT (CRRT) for patients with hemodynamic instability.6 Moreover, large-scale intensive care unit (ICU) clinical investigations show that CRRT has become the preferred treatment for AKI patients with unstable hemodynamics, regardless of sepsis status.7 The pathophysiological mechanism of SA-AKI is incompletely understood. However, recent research suggests that dysregulated immune responses in sepsis trigger a cascade of inflammatory reactions, leading to a “cytokine storm” that accelerates SA-AKI progression.8 In addition to conventional CRRT, various novel extracorporeal blood purification therapies targeting endotoxins and cytokines have emerged. These approaches aim to regulate immune-inflammatory responses by selectively removing circulating endotoxins and cytokines. Current extracorporeal blood purification techniques for SA-AKI include CRRT, high cut-off filters, hemoperfusion/hemoadsorption, highly adsorptive hemofiltration, and continuous plasma filtration adsorption.9
The Acute Dialysis Quality Initiative consensus distinguishes “sepsis-associated acute kidney injury” from “sepsis-induced acute kidney injury (SI-AKI)” to better characterize renal complications arising from sepsis.10 SA-AKI broadly encompasses cases where AKI occurs in the setting of sepsis, reflecting the interplay between infection, systemic inflammation, and renal dysfunction. In contrast, SI-AKI specifically refers to kidney injury predominantly caused by sepsis-related pathophysiological derangements, such as systemic inflammatory responses and microcirculatory dysfunction.11 Understanding these distinctions is crucial, given the heterogeneous nature of sepsis, which manifests in various phenotypes influenced by patient-specific factors such as genetic predisposition, comorbidities, and pathogen characteristics.12 This diversity underscores the need for precision medicine approaches, which aim to tailor interventions based on individual sepsis phenotypes and their unique pathophysiological mechanisms, thereby optimizing treatment efficacy and improving patient outcomes.3 Precision medicine in SA-AKI and SI-AKI is expected to refine therapeutic strategies and improve management of these complex conditions by addressing the specific needs of diverse patient populations.
Epidemiology of SA-AKI
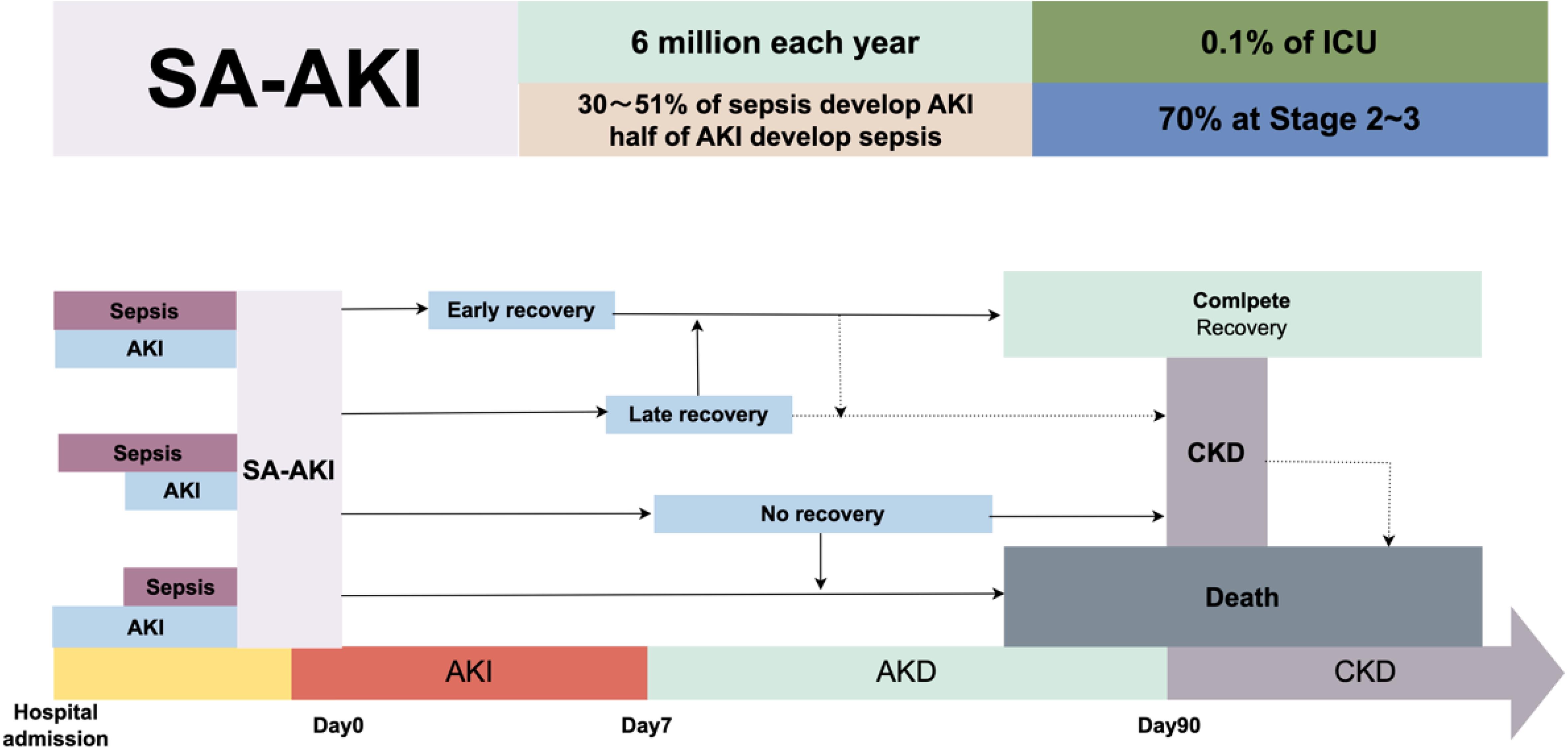
To date, very little is known about the epidemiology of SA-AKI.13 Clinically, about 30% of sepsis patients develop AKI, with a global incidence of SA-AKI estimated at around six million cases per year, or nearly 0.1%. In the ICU, almost half of the patients with AKI develop sepsis.9 A prospective cohort study involving 1,177 sepsis patients from multiple ICUs in Europe showed an AKI incidence of 51% and a mortality rate of 41%.14 A multicenter retrospective study found the incidence of AKI in sepsis patients to be 47.1%. This study included 1,243 patients with septic shock, of whom 50.4% were admitted to the emergency room due to AKI, and another 18.7% developed AKI within seven days.15 Clinically, 70% of AKI patients are classified as Stage 2 or 3. AKI is also prevalent among patients without severe sepsis or septic shock. Approximately 34% of non-severe community-acquired pneumonia patients develop AKI.16 These epidemiological data suggest a gradually increasing trend in the incidence of SA-AKI, which warrants greater research attention.17
Causes of SA-AKI
AKI in the context of SA-AKI represents a critical area of research due to its significant impact on patient morbidity and mortality rates in intensive care settings. SA-AKI is a common complication in septic patients, characterized by a sudden decline in renal function, which contributes to prolonged hospital stays, increased healthcare costs, and higher mortality rates.18 Understanding the pathophysiological mechanisms and risk factors associated with SA-AKI is crucial for developing targeted interventions, improving patient outcomes, and enhancing critical care protocols. Current research underscores the complex interplay between systemic inflammation, hemodynamic instability, and cellular injury in the progression of SA-AKI, emphasizing the need for early detection and personalized treatment strategies.19 Addressing the causes and consequences of SA-AKI not only aids in the development of preventative measures but also enhances the overall management of sepsis, contributing to broader healthcare objectives of reducing the global burden of critical illnesses.
Diagnosis of SA-AKI
The exact onset of sepsis and AKI is often difficult to pinpoint. Both sepsis and its treatments can cause kidney damage.3 If AKI is directly caused by sepsis, it is termed sepsis-induced AKI, which is usually considered a subphenotype of SA-AKI. However, the interaction between sepsis and AKI is complex, with sepsis potentially causing AKI and critically ill patients possibly developing sepsis following AKI—suggesting that AKI may increase the risk of sepsis.20 As such, the causality of SA-AKI remains to be fully determined. Moreover, diagnosing AKI based solely on serum creatinine and urine output has its limitations: sepsis can reduce peripheral blood perfusion, decreasing muscle blood supply, which reduces creatine production and delays an increase in serum creatinine levels, hindering early AKI detection. Inadequate urine output can also be diluted by aggressive fluid resuscitation, preventing timely diagnosis of AKI; the use of diuretics may further interfere with AKI diagnosis.21 Urine microscopy is a routine method for detecting kidney diseases. Compared to AKI patients caused by other factors, urine microscopy scores are higher in SA-AKI patients. When the urine microscopy score is ≥3, its specificity reaches up to 95%, but its sensitivity for detecting deteriorating AKI is relatively low (67%).6 This suggests that urinalysis may help determine the cause of AKI and provide prognostic information, but its sensitivity in diagnosing AKI and worsening AKI is not high.
Traditional biomarkers, such as serum creatinine, have limitations in the early diagnosis of acute kidney injury. In recent years, novel biomarkers like TIMP2IGFBP7 have been proposed for early AKI prediction and diagnosis. TIMP2IGFBP7 assesses the risk of kidney injury by detecting the product of TIMP2 and IGFBP7 levels in urine, demonstrating strong predictive performance across various clinical settings.22,23 As a cell cycle arrest biomarker, the TIMP2IGFBP7 concentration product in urine has been extensively studied for early AKI diagnosis. Studies indicate that TIMP2IGFBP7 has high sensitivity and specificity for predicting AKI in diverse clinical contexts. For instance, in a prospective study of 170 patients, the area under the curve for TIMP2IGFBP7 was 0.82, reflecting its robust predictive capability.24 Another study found that TIMP2IGFBP7 exhibited high sensitivity and specificity for predicting AKI following cardiac surgery, with a threshold value of 0.265 (ng/mL)∧2/1,000.25 In addition to TIMP2IGFBP7, other novel biomarkers have also shown potential in early AKI diagnosis. Urinary biomarkers such as neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, and liver-type fatty acid-binding protein have demonstrated good performance in early AKI prediction.23 The combined use of these biomarkers can further enhance the accuracy of AKI predictions. Despite promising results from TIMP2IGFBP7 and other novel biomarkers, their clinical application faces several challenges. Firstly, the performance of these biomarkers can vary depending on the clinical setting and patient population. For instance, while TIMP2*IGFBP7 performed well in predicting AKI post-cardiac surgery in some studies, its effectiveness may vary in others.26 Secondly, detecting these biomarkers requires specific equipment and techniques, limiting their application in resource-constrained settings. Furthermore, the critical thresholds and optimal timing for biomarker testing require additional research and validation.23
Course of SA-AKI
The precise onset of SA-AKI is currently unclear. In patients with sepsis, the existence of AKI should be suspected; conversely, the presence of sepsis should be considered in AKI patients (Fig. 1). AKI can occur simultaneously with sepsis at admission or manifest during hospitalization. Even when AKI is not present at the time of admission in septic patients, optimal resuscitation and appropriate sepsis treatment can still act preventatively against AKI.21 Once diagnosed with SA-AKI, there should be an emphasis on thorough monitoring and timely organ-supportive treatment to prevent further renal injury. SA-AKI may reverse within the first week of admission, indicating a generally positive prognosis.27 However, some patients may experience one or more episodes of AKI, highlighting the importance of continuous monitoring and avoidance of nephrotoxic injury, even if initial AKI has been reversed or recovered. Even patients who fully recover from SA-AKI remain at risk of developing CKD and other outcomes, including recurrent sepsis.28 Patients not fully recovered from AKI within seven days will be categorized as having acute kidney disease, which may later recover or progress to CKD and is associated with a poor long-term prognosis.29 Post-discharge survivors of SA-AKI should be followed up by nephrologists for continued management to monitor the occurrence of CKD and other adverse outcomes.
Sepsis-associated acute kidney injury (SA-AKI) presents a significant disease burden, with approximately 6 million cases reported annually. Nearly half of sepsis patients develop SA-AKI, and among those in the intensive care unit (ICU), the incidence is even higher, affecting 0.1% of ICU patients. Moreover, 70% of ICU patients with SA-AKI meet stage 2–3 severity criteria according to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines. This flowchart illustrates the potential progression and outcomes of SA-AKI. Upon hospital admission, patients present with SA-AKI without a clearly defined sequence of preceding sepsis or AKI events. Following admission, SA-AKI patients may follow one of three pathways over time: (1) early recovery of kidney function, (2) late recovery of kidney function, or (3) no recovery of kidney function. The third pathway, where kidney function does not recover, may further progress to acute kidney disease (AKD) or chronic kidney disease (CKD) or result in death. The flowchart depicts these three potential trajectories for SA-AKI patients, starting from the point of hospital admission when AKI is first identified.
Pathophysiological mechanism of SA-AKI
The pathophysiological mechanism of SA-AKI is still not completely understood. It is generally believed that SA-AKI is due to a reduction in bilateral renal glomerular blood perfusion, resulting in acute renal tubular ischemia and even apoptosis of renal tubular epithelial cells (RTEC). Additionally, inflammatory responses, metabolic adaptations, and microcirculatory dysfunction play significant roles in the organ damage phase of sepsis.29
Ischemia/reperfusion (I/R) injury
Hypoperfusion and shock are primary causes of AKI, with I/R injury leading to acute tubular necrosis and extensive cell death. However, during SA-AKI, various mechanisms operate beyond I/R injury.30 For instance, SA-AKI may occur even when renal hemodynamics are stable.
Inflammatory response
The inflammatory response is a defense mechanism that forms after a pathogen invades the host. During sepsis, inflammatory mediators such as pathogen-associated molecular patterns and damage-associated molecular patterns are released into the bloodstream.31 These molecules bind to pattern recognition receptors, such as Toll-like receptors (TLRs), on immune cells, triggering a downstream signaling cascade that leads to the synthesis and release of pro-inflammatory molecules. RTECs also express TLRs, specifically TLR2 and TLR4.32,33 Upon pathogen-associated molecular pattern activation, proximal RTECs exhibit increased oxidative stress, produce reactive oxygen species, and cause mitochondrial damage. There is evidence that RTECs initiate signal transduction through paracrine secretion.34 Furthermore, pathological observations revealed increased monocyte infiltration in the glomeruli and peritoneal regions of sepsis animal models, confirming the role of inflammation in AKI.35
Microcirculatory dysfunction
Studies have shown that even with stable renal blood supply, sepsis patients can still experience alterations in renal microcirculation.36 Features of SA-AKI include changes in the homogeneity of microcirculatory blood flow, manifested as a reduced proportion of capillary blood flow and an increased proportion of stalled flow.37 Multiple mechanisms may cause microcirculatory dysfunction, such as sympathetic and parasympathetic nervous system responses, endothelial injury, sloughing of the glycocalyx, and activation of the coagulation cascade. Endothelial injury reinforces leukocyte infiltration and platelet adhesion, while slowing blood flow, leading to capillary occlusion, microthrombus formation, and prolonged exposure of RTEC to activated inflammatory mediators. The increase in vascular permeability and impaired dilation function related to endothelial injury results in perivascular interstitial edema.38 This alters perfusion to the RTEC by increasing the oxygen diffusion distance from the capillaries to the RTEC and elevating the output pressure of the vein. Changes in microcirculatory hemodynamics may also play a key role in SA-AKI. The glomerular filtration rate is determined by intraglomerular hydrostatic pressure and is independent of renal blood flow changes; hence, the mechanism of afferent arteriolar constriction and efferent arteriolar dilation is suggested to explain the decrease in glomerular pressure, which results in reduced glomerular filtration rate.28
Additionally, during AKI, renal blood flow undergoes redistribution, diverting away from the medulla, bypassing the glomerulus, and directly connecting the incoming substances to the efferent capillaries of the arteriole. This partially explains blood shunting during SA-AKI. However, it remains unclear how or when these shunting pathways open. In summary, the increase in shunting and redistribution of blood flow explains the possible presence of mechanisms beyond ischemia during SA-AKI.39
Metabolic adaptation
During SA-AKI, metabolic adaptation can occur, where a method is adopted that maintains the minimum function of cells and organs to ensure cell survival. Various theories have been proposed to explain the metabolic adaptation of RTEC cells during sepsis, most of which are mitochondria-mediated and characterized by optimized energy consumption. Inflammation is associated with optimized energy consumption, implying a reduction in the efficiency of energy utilization in protein synthesis or ion transport, thereby maintaining only energy use for crucial cell functions and simultaneously avoiding cell death.40 During inflammation, the expression of renal tubule transporter proteins is down-regulated, and renal tubule ion transport is reduced, suggesting that metabolic adaptation can re-determine priority in energy consumption, which is an adaptive mechanism.41 The exact mechanisms of how metabolic adaptation occurs are still unclear.
Lipid metabolism alterations
The alterations in lipid metabolism observed in sepsis are complex and multifaceted, affecting not only the progression of the disease but also potentially providing new therapeutic targets.42 Sepsis is a systemic inflammatory response syndrome triggered by infection, characterized by an excessive host response that leads to multi-organ dysfunction. In patients with sepsis, there are significant changes in lipid metabolism, particularly a decrease in high-density lipoprotein (HDL) levels, which are closely linked to the exacerbation of the inflammatory response and endothelial dysfunction.43 The role of HDL in sepsis extends beyond cholesterol transport, encompassing anti-inflammatory, anti-apoptotic, antithrombotic, and anti-infective properties. During sepsis, both the serum levels and composition of HDL are significantly altered, potentially leading to dysregulation of host immune responses and endothelial dysfunction. Research indicates that the anti-inflammatory capacity of HDL is suppressed in sepsis, possibly due to the release of inflammatory mediators and increased oxidative stress. Furthermore, changes in lipid metabolism may influence the prognosis of sepsis. For instance, low HDL levels are associated with poor outcomes in sepsis patients, suggesting HDL’s potential as a biomarker for predicting sepsis severity and prognosis. Additionally, some studies have explored the potential of treating sepsis by modulating lipid metabolism.42,43
Treatment of SA-AKI
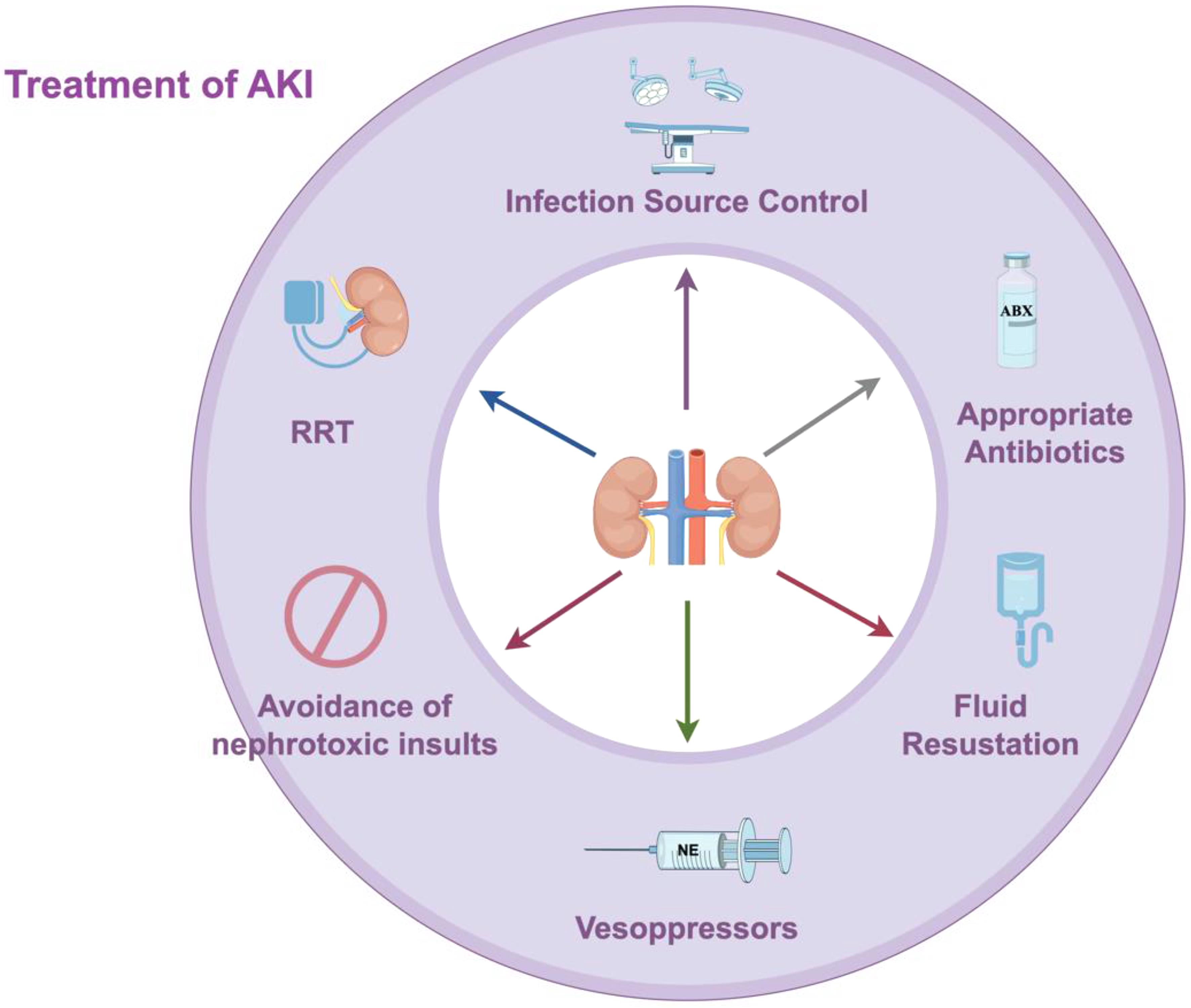
Control of the pathogenesis and early, appropriate use of antimicrobial drugs remain vital in the treatment of sepsis and can also prevent further renal injury. Delay in the administration of antimicrobial drugs is associated with the early development of AKI. However, caution should be exercised when using certain nephrotoxic drugs involved in treatment, such as aminoglycosides and vancomycin. The KDIGO guideline suggests that diagnostic drugs such as amphotericin B and intravenous radiographic contrast agents should be used cautiously to avoid renal injury, with strict drug monitoring considered when necessary.6 A bundled treatment approach for SA-AKI may be beneficial, including infection source control, appropriate antibiotics, fluid therapy, vasopressors, avoidance of nephrotoxic insults, and RRT (Fig. 2).
This diagram outlines a bundled management approach for sepsis-associated acute kidney injury (SA-AKI). The bundle consists of six key interventions: (1) infection source control, (2) appropriate antibiotic use, (3) fluid therapy, (4) vasopressor administration, (5) avoidance of nephrotoxic insults, and (6) renal replacement therapy (RRT).
Fluid resuscitation
The use of vasoactive medications after fluid resuscitation forms the foundation of shock treatment. Three landmark clinical trials conducted among septic shock patients have consistently indicated that resuscitation strategies do not confer advantages in terms of reducing mortality rates or the need for RRT.5 Additionally, the influence of early goal-directed therapy, alternative resuscitation strategies, or conventional therapy on the development of new AKI, the severity of AKI, fluid overload, the need for RRT, or renal recovery is minimal.44
Types of resuscitation fluids
Every type of intravenous fluid infusion has the potential to lead to fluid overload and renal burden. A prospective observational study indicated that patients with AKI had a greater cumulative fluid intake in the first three days, suggesting that fluid overload can function as an independent risk factor for the severity of AKI. Evidence demonstrates that the use of hydroxyethyl starch elevates the incidence of AKI, the need for RRT, and the risk of mortality, especially when administered in large quantities to patients with sepsis.14 In contrast to crystalloids, gelatin may correlate with a higher occurrence rate of AKI and a greater requirement for RRT among septic patients.45 Albumin solution is usually considered safe for sepsis resuscitation; however, recent observational research involving shock patients revealed a significant correlation between early, substantial albumin infusion post-surgery and the development of AKI.46
An observational study involving ICU patients, including those experiencing septic shock, demonstrated a reduced incidence and mortality rate of AKI when using Ringer’s solution. Notably, even within sepsis subgroups, there was no statistically significant difference in AKI incidence or the proportion requiring RRT when comparing patients treated with normal saline to those treated with balanced crystalloid solutions.47 Other reports indicate that the risk of major adverse renal events is reduced among patients treated with balanced crystalloid solutions.48 Therefore, balanced solutions should be used instead of normal saline, particularly in patients with sepsis.49
Vasoactive drugs
Norepinephrine is the drug of choice for the treatment of septic shock. Dopamine, compared to norepinephrine, is associated with more adverse events and is not recommended for renal protection.50 Vasopressin is associated with a lower rate of RRT and does not appear to increase the risk of AKI.51 A large randomized controlled trial for septic shock showed that a blood pressure level of 80–85 mmHg (1 mmHg ≈ 0.133 kPa) reduced the need for RRT in patients compared to a target mean arterial pressure of 65–70 mmHg, but no survival benefit was observed.52
Initiation timing of renal replacement therapy
Early application of RRT has been shown to significantly benefit patients with severe AKI within 90 days of initiating RRT.46 Concurrently, early treatment substantially reduces the incidence of major adverse kidney events and mortality within a year, contributing to the restoration of kidney function.53 However, the findings of a trial comparing the effectiveness of early versus delayed dialysis in patients with septic shock and severe AKI in the ICU demonstrated no statistically significant difference in mortality rates between these two RRT approaches.54 Alarmingly, 9% of patients died regardless of whether early or delayed initiation was used.53 The Standard versus Accelerated Initiation of Renal-Replacement Therapy in Acute Kidney Injury trial investigated the optimal timing for initiating kidney replacement therapy in patients with acute kidney injury. The trial compared two strategies: accelerated initiation versus standard initiation of KRT. The findings revealed a neutral outcome concerning 90-day all-cause mortality, indicating that early initiation of KRT does not confer additional survival benefits compared to the standard initiation strategy in critically ill patients.55 The appropriate timing for the initiation of RRT is still under debate.
Blood purification
Clinical evidence on blood purification remains limited, with most studies not having measured target solutes. It is still unclear whether inflammatory mediators can be removed, and even if they can, whether their removal benefits the prognosis or survival. In patients with septic shock, cytokine levels are highly variable, and high endogenous clearance rates make their levels dynamic.56 The EUPHRATES trial evaluated the efficacy of using a blood purification device in patients with severe sepsis and septic shock. While the primary outcome did not demonstrate a significant improvement in overall survival rates, potential benefits were observed in certain subgroups of patients.57 Furthermore, the development of novel therapies based on adsorption devices has drawn attention to the clearance of specific mediators involved in organ dysfunction and antibiotic removal. The joint commission of the Italian Society of Anesthesiology and Critical Care and the Italian Society of Nephrology has addressed some of these issues, proposed clinical practice recommendations, and developed a framework for future research in this field.58
High-volume hemofiltration (HVHF), defined as a convective dose of >35 mL·kg−1·h−1, has been explored in several studies regarding its efficacy in treating SA-AKI patients, with varying results in mortality rates. A multi-center randomized controlled trial involving 140 SA-AKI patients, divided into two groups receiving either HVHF at a rate of 70 mL·kg−1·h−1 or standard hemofiltration at 35 mL·kg−1·h−1 for 96 h, demonstrated no significant statistical difference in patient mortality at 28, 60, and 90 days between the two treatment methods.59 Another randomized controlled trial comparing the treatment effects of varying dosages of HVHF in SA-AKI patients also showed no significant statistical difference in mortality or renal outcomes between patients treated with 85 mL·kg−1·h−1 and 50 mL·kg−1·h−1 HVHF.60 Additionally, a meta-analysis incorporating four HVHF trials involving SA-AKI patients indicated no benefit in 28-day survival rates and an increased incidence of hypophosphatemia and hypokalemia.61
Polymyxin B hemoperfusion (PMX-HP), utilized as an adjunctive measure in sepsis treatment, can remove endotoxins from the blood circulation. Various randomized controlled trials have confirmed the efficacy of PMX-HP in sepsis, though some studies have yielded contrary findings. A European multi-center pilot study involving 36 intra-abdominal sepsis surgical patients demonstrated that a 2-h PMX-HP treatment could only improve left ventricular function and decrease the need for RRT.62,63 Research that included 64 severe intra-abdominal sepsis patients showed benefits of PMX-HP treatment in terms of hemodynamics, organ function, and 28-day survival rate.64 However, a subsequent larger randomized controlled trial yielded negative results.65 Additionally, the immunomodulatory role of PMX-HP was reported in another randomized controlled trial, validating its ability to enhance the recruitment of monocytes and neutrophils in sepsis patients, but without any benefit in reducing mortality or improving renal outcomes.66
Prognosis of SA-AKI
SA-AKI is closely associated with poor clinical outcomes, including acute kidney disease, CKD, or death (Fig. 1). In critically ill patients with AKI, those with SA-AKI have a higher risk of in-hospital death and a longer hospital stay.9,67 In patients with SA-AKI that reverses within 24 h post-shock, there is a reduced in-hospital mortality rate. The long-term prognosis of SA-AKI patients depends on the primary disease, the severity of AKI, and the baseline condition at discharge. Patients who recover from AKI have significantly improved survival rates, but there remains a possibility of CKD, end-stage renal disease, and death. In a follow-up of over a year for 105 survivors, it was found that the rates of CKD in patients with reversed AKI, recovered AKI, and unrecovered AKI were 21%, 30%, and 79%, respectively.68 Another long-term follow-up study of 19,276 survivors showed that the 15-year mortality rates of suspected SA-AKI by renal recovery status at discharge were 57.1% for no recovery, 47.9% for recovery, and 36.5% for no AKI.54 The severity of AKI, timely treatment, and recovery status during hospitalization are critical factors in determining the prognosis.69
Conclusions
In summary, one-third of sepsis patients develop AKI, with half of these cases being severe. Close monitoring and timely treatment are crucial for SA-AKI to prevent further kidney injury and improve prognosis. The main mechanisms include reduced renal perfusion, inflammation, and impaired microcirculation. Effective sepsis treatment involves addressing the cause and using antibiotics, fluid resuscitation, and vasopressors appropriately. Research is advancing in finding biomarkers for early SA-AKI diagnosis, such as neutrophil gelatinase-associated lipocalin and kidney injury molecule-1, which may improve future diagnostics. Further studies, particularly on the timing of RRT, are essential to enhance treatment outcomes.
Declarations
Acknowledgement
We acknowledge Figdraw (
Funding
None.
Conflict of interest
One of the authors, Linlin Zhang, serves as an editorial board member of Nature Cell and Science. The authors declare no other conflicts of interest.
Authors’ contributions
Conceptualization: L.Z.; Project administration: L.Z; Resources: N.X.; Visualization: N.X.; Writing – original draft: N.X.; Writing – review & editing: L.Z.

 Author information
Author information