Introduction
The microbiota is a complex ecosystem that has garnered significant attention due to its impact on human health and disease. Ongoing research continues to elucidate its interactions with other bodily systems. The microorganisms that comprise this ecosystem are commonly found in various parts of the human body, such as the eyes, skin, mouth, and gastrointestinal system.1 Among these, the gastrointestinal tract harbors more than 100 trillion microorganisms, making it the ‘main settlement organ’ of the microbiota.1 Given that bacterial cell density in the intestines ranges from 1011 to 1012 per gram, factors influencing this area are believed to have significant effects on the human body.
The microbiota plays a crucial role in maintaining physiological homeostasis. Its effects on host physiology include enhanced energy extraction from food, modulation of appetite signals, metabolism of undigested carbohydrates, and the biosynthesis of vitamins and amino acids.2 Beyond these functions, the microbiota acts as a physical barrier protecting the host from pathogens, while also stimulating and maturing the immune system and epithelial cells. Furthermore, the gut microbiota appears to modulate excitatory and inhibitory neurotransmitters, such as serotonin, GABA, and dopamine, and neurotransmitter-like substances, particularly in response to physical and emotional stress.3,4
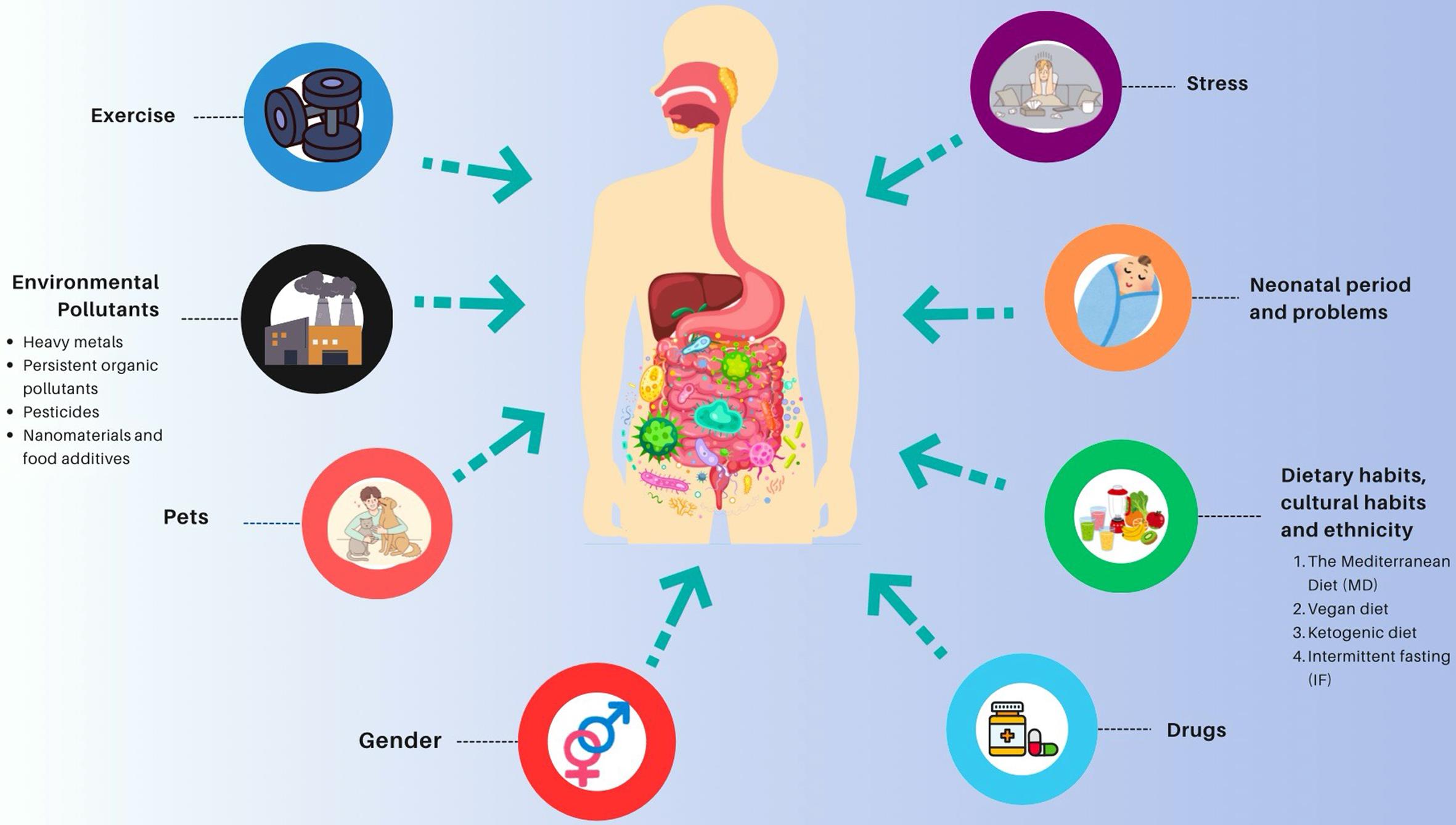
The microbiota begins to colonize various regions of the human body shortly after birth; however, only some of these microorganisms will remain and persist over time. The microbiota is a living, dynamic environment that can change daily, weekly, and monthly. This environment is sensitive to many external factors, including the host’s age, medications, dietary habits, exercise, stress, and environmental pollutants (Fig. 1).5 This review aimed to examine the factors that affect the microbiota in the human body, particularly in the intestines, and their potential consequences.
Change of microbiota variations in individuals over time
Humans host vastly different collections of microbes with varying densities, and these variations can differ between individuals and over time. However, the causes and regulation of these differences are not yet fully understood. Additionally, the precise impact of microbiota differences on wellness, health maintenance, and the onset and progression of disease remains largely unknown. The development of the microbiota begins in the early postnatal period, with a predominance of facultative anaerobes such as Streptococcus, Enterobacter, and Streptococcus. This initial population is later replaced by obligate anaerobes, including Bifidobacterium, Bacteroides, Prevotella, Ruminococcus, Clostridium, and Escherichia.6 By the age of one, a child’s microbiota is characterized by a higher abundance of Akkermansia muciniphila, Bacteroides, Veillonella, Clostridium coccoides spp., and Clostridium botulinum spp.5
The shaping of a child’s microbiota is nearly complete within the first three years of life.7 Studies indicate that three bacterial phyla, Firmicutes, Bacteroidetes, and Actinobacteria, are dominant during adulthood.7
In individuals over 70 years of age, the microbiota composition is influenced by a weakening immune system and changes in digestion and nutrient absorption. Studies conducted in this age group have shown a decrease in anaerobic bacteria, such as Bifidobacterium spp., and an increase in Clostridium and Proteobacteria. The reduction of Bifidobacterium spp. may lead to a low-grade systemic inflammatory state and malnutrition.8
Although the age-related development of the microbiota is well-documented, there is a lack of studies addressing the contribution of gender, and the results of those that do exist are contradictory. For example, an analysis of a study conducted on 91 individuals from France, Denmark, Germany, the Netherlands, and the United Kingdom found no significant difference in colon microbiota between genders. In contrast, a 2006 study conducted by four centers in France, Germany, Italy, and Sweden found higher levels of the Bacteroides-Prevotella group in males, but no difference in abundance between males and females when two species-specific probes targeting Bacteroides vulgatus and Bacteroides putredinis were used.9 The study emphasized that the results of gender-related research are contradictory and require detailed analysis with larger sample sizes.
Factors affecting microbiota variations
Neonatal period and problems
The colonization of a newborn’s microbiota is influenced by various environmental factors experienced from birth, such as the week of birth (prematurity/maturity), mode of delivery (vaginal birth/cesarean section), place of birth (home/hospital), hospitalization, medication, and diet (breast milk/formula). The microbiota of premature infants differs from that of full-term infants. Specifically, the intestinal microbiota of premature infants contains higher levels of Enterobacteriaceae, Enterococci, and Staphylococci, while Bifidobacteriaceae and Lactobacilli are less abundant.10 This difference is attributed to various factors, including hospitalization due to organ immaturity, antibiotic use, lack of breastfeeding, and parenteral nutrition.10 Premature birth can affect the maturation of the postnatal intestine and the development of systemic immunity.11 Additionally, premature infants may experience delayed colonization and reduced microbiota diversity due to a thin intestinal mucus layer, which can result in feeding intolerance, an increased risk of neonatal necrotizing enterocolitis, various intestinal infections, and ultimately higher morbidity and mortality rates.12 Supporting intestinal immunity holds great promise for improving the health of premature infants by reducing their risk of morbidity and mortality.
Mode of delivery is another determinant of microbiota diversity. During vaginal births, babies are exposed to Lactobacillus, Prevotella, and Sneathia from the mother’s genital tract.13 This colonization contributes to the digestion of milk.14 Babies delivered by cesarean section are often colonized with bacteria such as Staphylococcus, Propionibacterium, and Corynebacterium, which are typically present on the skin and are transmitted by healthcare workers’ hands.15 Cesarean delivery has been associated with an increased risk of certain disorders, including asthma, juvenile arthritis, inflammatory bowel disease, and obesity.16,17
Microbiota colonization also differs depending on whether the birth takes place at home or in a hospital. Hospital births are associated with a decrease in Bacteroides, Bifidobacterium, and Ruminococcus, and an increase in Enterobacteria and Clostridium species.5
Breastfeeding can provide infants with a more beneficial gut microbiota, characterized by a higher presence of Bifidobacterium species and lower levels of Clostridium difficile and Escherichia coli. Bifidobacterium helps produce short-chain fatty acids from breast milk. Furthermore, breast milk has been shown to protect newborns against intestinal dysfunction and inflammation by promoting immunity through oligosaccharides, immunoglobulins, and lactoferrin.18 Lactoferrin plays a crucial role in the immunological maturation of premature infants by influencing the colonization and development of beneficial gut bacteria.6,18
After breastfeeding ceases and solid foods are introduced, the gut microbiota becomes dominated by the genera Bifidobacterium, Clostridium coccoides, and Bacteroides. The consumption of high-fiber and carbohydrate-rich foods leads to an increase in Firmicutes and Prevotella, while the consumption of animal protein leads to an increase in Bacteroidetes. These variations are influenced by cultural dietary habits.6
Currently, several practices, including the increase in cesarean delivery rates, perinatal antibiotic use, and formula feeding, can disrupt the transmission and colonization of the newborn’s microbiota. These practices may also decrease the overall diversity of the infant’s microbiota and contribute to the development of drug-resistant organisms.
Dietary habits, cultural habits, and ethnicity
Dietary habits change with age and are culturally shaped by social and regional differences. An individual’s dietary choices may be influenced by concerns related to body mass index or lifestyle preferences, such as adhering to a strict vegan diet. Additionally, the level of societal development, such as industrialization, and cultural differences also play a role in shaping dietary habits. The quality, type, and source of food shape the gut microbiota, influencing its function and interactions with host microbes.6
The Western diet is characterized by high consumption of red meat, saturated fats, sugars, and processed foods. This type of diet may lead to a reduction in certain bacteria associated with anti-inflammatory states, such as Akkermansia muciniphila, Faecalibacterium prausnitzii, and Clostridium clusters XIVa and IV.9
Dietary fatty acids have also been found to influence the gut microbiota. A high-fat diet can result in the production of endotoxins (or lipopolysaccharides) by the gut microbiota, which may create an ideal environment for the growth of gram-negative bacteria, such as Enterobacteriaceae.19 This increase in endotoxin levels can cause inflammation and alter the composition of the gut microbiota, affecting intestinal permeability and promoting obesity.
The Mediterranean Diet (MD) is a plant-based diet that includes daily servings of vegetables, fruits, cereals, and olive oil.20 This dietary style, combined with the lifestyle and culture of the Mediterranean, is associated with a longer lifespan, a reduced risk of diseases (e.g., diabetes mellitus, obesity, and malignancy), and improved cognitive function. The gut microbiota changes observed with the MD include an increase in species that produce short-chain fatty acids, such as Clostridium leptum and Eubacterium rectale, an increase in Bifidobacteria, Bacteroides, and Faecalibacterium prausnitzii, and a decrease in Firmicutes and Blautia species.21 These changes are positively associated with inflammatory and oxidative status, malignancy risk, and overall metabolic health.21 The gut microbiota of individuals following the MD is characterized by increased levels of the Prevotella genus and Faecalibacterium prausnitzii, whereas those following the Western diet exhibit increased levels of Bacteroides.
A vegan diet involves consuming a high amount of fruits and vegetables while limiting the intake of saturated fats and excluding all animal products. In contrast, an omnivorous diet includes both animal and plant products. A study comparing the microbiota of individuals following vegan and omnivorous diets found that their microbiota were surprisingly similar.19
Individuals who choose to exclude gluten from their diet have been found to experience a decrease in intestinal dysbiosis and a reduction in the levels of several important microbial types.22 However, it is important to note that this dietary choice may also increase the risk of heart disease.23 On the other hand, this diet has been shown to reduce the symptoms of irritable bowel syndrome by decreasing the proportion of Bifidobacterium in patients, as demonstrated in randomized controlled trials.24
The ketogenic diet has gained popularity in recent years. It is a low-carbohydrate diet that aims to increase fat metabolism through hepatic ketogenesis, resulting in the production of ketone bodies. This diet is primarily used in the therapeutic treatment of neurological disorders, including Alzheimer’s disease, Parkinson’s disease, depression, and epilepsy.25 Although the ketogenic diet has been shown to affect the intestinal microbiota in animal studies, it is unclear which taxa are most affected, and the results are contradictory.
Intermittent fasting, also known as a time-limited diet, can enhance cellular defenses against oxidative and metabolic stress during fasting and activate pathways to eliminate or repair damaged molecules. Mouse studies have shown that intermittent fasting from a young age can prolong life expectancy by up to 80%. Studies have also shown that fasting and calorie control can modify gut flora, enhance insulin sensitivity, decrease the expression of inflammatory factors associated with lipid metabolism, and thereby prevent metabolic diseases.26 Dietary patterns and contents have become more complex with the addition of popular concepts such as prebiotics, probiotics, and synbiotics. Probiotics, such as yogurt, are non-pathogenic microorganisms naturally present in the human digestive system, including Lactobacilli, Bifidobacteria, and Enterococci. Prebiotics are non-digestible food ingredients that benefit the host by supporting the growth and/or activity of beneficial bacteria.27 Prebiotics are composed of indigestible carbohydrates, such as lactulose, inulin, and oligosaccharides. Numerous studies have demonstrated the significant effects of probiotics and prebiotics on human gastrointestinal health, particularly in the treatment and prevention of gastrointestinal infections. However, it is challenging to compare the results of these studies due to differences in factors such as the type of probiotic microorganism used, dosage, human populations studied, or the in vitro versus in vivo nature of the study.
In recent years, various dietary habits have become increasingly common, including increased consumption of processed foods and high-intensity sweeteners, as well as strict vegan, ketogenic, and gluten-free diets. It is important to consider the effects of these dietary patterns on the microbiota and their potential consequences. This issue will be discussed in the food additives section.
Studies have shown that each dietary change is accompanied by changes in the gut microbiota and an increase in the corresponding genes. This is particularly evident when an infant transitions to a fully adult diet, as an increase in genes associated with vitamin biosynthesis and polysaccharide digestion is detected in the microbiome.26,28
Food additives
A notable shift in contemporary dietary patterns is the growing consumption of processed foods and high-intensity sweeteners. These products are designated as food additives and are used to enhance the color, taste, odor, nutritional value, and shelf life of food products. The term “food additives” encompasses a diverse range of substances, including flavor enhancers, antioxidants, preservatives, sweeteners, and emulsifiers.29 They can be derived from natural or synthetic sources.
Flavor enhancers are amino acids and nucleotides commonly used to improve the taste of foods. Monosodium glutamate is the most prevalent flavor enhancer employed in the processing of food products. In 2017, Peng et al. examined the gut microbiota of 12 volunteers who consumed 2 g of monosodium glutamate per day for four consecutive weeks.30 The results demonstrated that the community structure and functional properties of the microbiota remained unaltered.
Antioxidants are a type of food additive used to extend the shelf life of food products by preventing oxidation. Natural antioxidants include tocopherols, while synthetic antioxidants include phenolic antioxidants.31 Antioxidants are extensively employed in the production of edible oils and fats. A study investigating the sensitivity of the human gut microbiota to phenolic compounds demonstrated that natural antioxidants (e.g., eugenol, ferulic acid, and vanillin) exhibited inhibitory effects on the growth of Agathobacter and Clostridium strains.32 In contrast, Bacteroidetes and Actinobacteria strains exhibited minimal sensitivity to phenolics. The bacteriostatic or bactericidal properties of natural antioxidants have been established. Given the paucity of research on synthetic antioxidants and the uncertainty surrounding their effects on the intestinal microbiota, further investigation is warranted.
The use of preservatives is intended to ensure the safety of foods and prevent loss of quality resulting from various reactions, including physical, chemical, or enzymatic processes. Some preservatives act as antioxidants. However, synthetic preservatives, including sodium benzoate, sodium nitrite, nitrite, and potassium sorbate, are considered to be of concern. A human study demonstrated that gut microbes with known anti-inflammatory properties, such as Clostridium tyrobutyricum or Lactobacillus paracasei, exhibited significantly greater sensitivity to additives than microbes with known pro-inflammatory or colitogenic properties, such as Bacteroides thetaiotaomicron or Enterococcus faecalis.33 It is postulated that these alterations in the intestinal microbiota may be partially attributable to the observed increase in allergic and autoimmune diseases. A study in mice showed that long-term exposure to nitrite was associated with obesity, diabetes, cardiometabolic diseases, and inflammation due to its impact on the reduction of Akkermansia.34
Sweeteners are sugar substitutes that imitate the sweet taste of sugar but have minimal impact on energy intake. They can be classified into two categories: nutritional sweeteners (polyols or sugar alcohols) and intense sweeteners, as well as synthetic and natural sweeteners.
The group of nutritional sweeteners includes high-fructose corn syrup, isomaltulose, and trehalose.35 These are not considered food additives; rather, they are classified as components. Polyols (e.g., erythritol, isomaltitol, lactitol, maltitol, sorbitol, mannitol, and xylitol) are considered food additives. Polyols are naturally occurring sweeteners found in fruits and vegetables. They have become the most consumed sweetener group because they do not have cariogenic properties, do not trigger salivation, and do not interfere with insulin levels.35
İntensity sweeteners are food additives used as an alternative to sugar due to their much sweeter taste. Sweeteners are used because of their negligible calorie content and high sweetening capacity. Steviol glycosides, thaumatin, and neohesperidin dihydrochalcone are the most common natural sweeteners.35 Well-known synthetic sweeteners include sucralose, aspartame, and saccharin.35 Some animal studies suggest that these synthetic sweeteners may have negative effects on the intestinal microbiota.36 One study found that sucralose decreased the presence of Bacteroides, Clostridia, and aerobic bacteria in rat intestines.37 There is a paucity of human studies on sucralose. The study by Thomson et al. (2019) was a randomized, double-blind study of sucralose in 34 healthy men. Sixteen subjects were administered a daily dose of 780 mg of sucralose for one week, while the control group received a placebo (n = 17). The results of this study demonstrated that the composition of the gut microbiome remained unaltered in healthy individuals at the phylum level.38
Non-caloric artificial sweeteners are often recommended for weight loss, glucose intolerance, or type 2 diabetes. However, a recent report showed that chronic feeding of mice with non-caloric artificial sweeteners, such as saccharin, sucralose, and aspartame, resulted in higher glucose intolerance.39 This was associated with an increased abundance of bacteria from the genus Bacteroides and the order Clostridiales in the gut.
Emulsifiers are food additives used to extend the shelf life and freshness of processed foods. However, studies have shown that they can have adverse effects on the intestinal microbiota and the integrity of intestinal tissue due to their detergent-like properties. These were found to be associated with symptoms of metabolic syndrome by causing microbiota alteration (increased abundance of Ruminococcus gnavus and decreased abundance of Bacteroidales in feces).39 Emulsifier-induced changes in the microbiota were found to be associated with symptoms of metabolic syndrome,40 including reduced intestinal mucus thickness, low-grade inflammation, increased adiposity, and glucose dysregulation.
Although food additives are used within safe limits, their health effects remain unclear. The research in this field is still in its infancy, with the majority of studies conducted on animals. Furthermore, the number of human studies is limited. Further studies are required to gain a more comprehensive understanding of the effects of these substances.
Drugs
Since the 1950s, drugs have been increasingly used in human and veterinary medicine. Recent studies have shown that this widespread use has led to high concentrations of drugs, particularly antibiotics, being found in natural environments such as river and lake waters, agricultural soils, and wastewater.41 The detection of drugs in natural environments indicates that organisms passively ingest these substances in various ways without realizing it.
Drugs can have various effects—both positive and negative—on human health, affecting various organs, systems, and even the microbiota. However, the relationship between drug use, microbiota, and the host is complex and multidimensional. Drug response varies between individuals due to several factors, including age, genetics, lifestyle, disease status, drug-drug interactions, environmental factors, and intestinal microbiota. Additionally, drugs can cause changes in the microbiota, which in turn affects drug response and toxicity. The interaction between microbiota and drugs, or drug-microbiota interaction, is based on two important points: (1) the effect of drug use on intestinal microbiota, and (2) the importance of microbiota in the metabolism of drugs.
The effect of drug use on intestinal microbiota
Medications, including laxatives, proton pump inhibitors, and antibiotics, have been shown to cause changes in the composition of the intestinal microbiota. Studies indicate that antibiotics, in particular, alter the overall diversity of the microbiota (either increasing or decreasing it) and contribute to the development of drug-resistant organisms. It has been proven that these effects can last for several years, up to four years in some cases.42 Some antibiotics may increase the severity of microbial-related diseases, such as Clostridium difficile-associated diarrhea. In contrast, some changes in the host microbiota due to antibiotics may reduce the severity of certain diseases (e.g., cirrhosis, cystic fibrosis), thus alleviating the clinical course. Furthermore, research has shown that early exposure to antibiotics is linked to an increased risk of developing allergic diseases, eczema, obesity, and type 1 diabetes.43
The importance of microbiota in the metabolism of drugs
The human gut microbiota contains enzymes that modify both systemically and orally administered drugs, leading to activation, inactivation, toxication, altered stability, poor bioavailability, and rapid excretion of drugs.44 These effects occur due to the microbiota’s impact on drug pharmacokinetics and pharmacodynamics, as well as its ability to trigger drug-drug interactions. The impact of microbiota on drug metabolism depends on the specific drug, drug combination, and individual genetics. This variability accounts for why therapeutic doses of the same drugs can be effective for some individuals but not for others.
Living with pets
Studies on pets, their owners, and their microbiota began in the 1980s, revealing that pets and their owners share common gut bacteria. In the 2000s, research on infants and children demonstrated that early exposure to furry pets at home increases the richness and diversity of the human gut microbiome and reduces the incidence of atopic and allergic diseases. Publications suggest that increasing the abundance of Ruminococcus and Oscillospira species may protect against allergic disorders and obesity in children.45
Environmental pollutants
Environmental pollutants are a growing public health concern closely linked to industrialization. The most common types of environmental pollutants are heavy metals, persistent organic pollutants, food additives, and pesticides. Detecting and controlling these pollutants is crucial for maintaining good health due to their toxic effects on living organisms.
Heavy metals
Arsenic (As), cadmium (Cd), and lead are heavy metals commonly found in the environment and have been extensively studied due to their potential harmful effects. These metals can be encountered in various aspects of daily life, both knowingly and unknowingly.
As is a known carcinogen that is widely present in our environment. Animal experiments conducted on mice have shown that exposure to As leads to a significant decrease in the abundance of Firmicutes and a significant increase in the abundance of Bacteroidetes.46 Reports suggest that exposure to As is associated with various diseases, including diabetes, cardiovascular disorders, and cancers.46
Cd is used in the production of various products, including batteries, metal plating, pigments, and plastics. High concentrations of Cd have been observed in water systems and soil in developing countries.47 Similar to arsenic, studies on mice have shown that cadmium exposure leads to a decrease in the abundance of Firmicutes and γ-Proteobacteria in the intestines, while Bacteroidetes increased. Cd toxicity is associated with carcinogenesis, hepatotoxicity, oxidative stress, and immunotoxicity.48
Lead is a toxic metal commonly found in various consumer goods, such as batteries and cable insulation. It does not have a reliable blood level. Studies have shown that lead exposure can cause a decrease in the ratio of Bacteroidetes to Firmicutes in the intestines, while Desulfovibrionaceae, Barnesiella, and Clostridium XIVb increased.46 In mice, lead poisoning has been found to disrupt energy production and other metabolic processes, potentially contributing to the development of obesity.49
Persistent organic pollutants
Organochlorine pesticides, polychlorinated biphenyls, polybrominated diphenyl ethers, and polycyclic aromatic hydrocarbons are persistent organic compounds.46 They are the most persistent of all known chemicals in nature. Their main effects are on the intestinal microbiota, as exposure is mostly through the ingestion of contaminated food and water. Persistent organic compounds have been reported as contributing factors to some common global diseases (such as obesity and diabetes) and developmental disorders.46
Polychlorinated biphenyls are lipophilic industrial compounds primarily used in the production of capacitors, transformers, coolants, hydraulic fluids, and lubricants to prevent combustion and energy loss.47
Exposure to polycyclic aromatic hydrocarbons occurs through the oral ingestion of charcoal-roasted, grilled, and smoked meats or the consumption of poorly cleaned vegetables.50 These chemical compounds have toxic, carcinogenic, and potentially estrogenic or antiestrogenic effects on humans.51
Pesticides
Pesticides are primarily detected in foodstuffs, water, and soil due to their prevalent use in agriculture. Commonly used pesticides include permethrin, pentachlorophenol, the azole fungicide epoxiconazole, chlorpyrifos, and imazalil.46 These pesticides serve various purposes in agriculture, such as treating fruits and vegetables and preventing fungal diseases. The impact of pesticides on the intestinal microbiota varies between different types. Animal studies have shown that they can lead to various health issues, including neurotoxicity, impaired liver function, oxidative stress, hydropic degeneration, reproductive toxicity, and endocrine disruption.46,49
Nanomaterials
Nanomaterials have a diverse range of applications, such as in biomedicine and diagnostic imaging, due to their superior physical and chemical properties.52 They are used as coatings to mitigate mechanical damage or microbiological contamination and to enhance the color and taste of food.53 Some nanomaterials have been demonstrated to possess antibacterial properties.54 It is therefore postulated that these materials may alter the intestinal microbiota and affect host health. Studies have observed that Firmicutes, the most common microbiota in the intestine, is particularly sensitive to nanomaterials. The results demonstrated differences in microbiota composition, with Firmicutes showing either an increase or decrease, while Bacteroidetes exhibited a decrease.54
Nanoparticles, nanocapsules, and nanofilms are among the most commonly utilized nanomaterials.55 Nanoparticles (NPs) are ultra-small in size, allowing them to enter the human body through various routes, including inhalation, ingestion, skin penetration, and injection. They have been found to induce oxidative stress, but some NPs (such as silver NPs) have also been shown to have cytotoxic effects on microorganisms like bacteria, viruses, and fungi, making them potentially useful as antimicrobial agents.56 However, results regarding their antimicrobial effects on the gut microbiota are conflicting, and more evidence is required.
Nanocapsules can be used as carriers for the introduction, protection, and transportation of active chemicals in foods and pharmaceuticals, while maintaining the appearance and taste of the product.54 Nanofilms are employed in a variety of food products due to their ability to protect food surfaces from moisture, oil, and gas.
It is regrettable that most research investigating the impact of nanomaterials on the gut microbiota is confined to animal experiments or in vitro tests. Data on actual exposure concentrations of nanomaterials are scarce and based on animal experiments. Future studies should focus on human research to provide more comprehensive insights.
Exercise
Regular physical exercise not only promotes physical health and appearance but also enhances mood by stimulating the production of endorphins. In addition to these well-known benefits, research has shown that physical exercise can increase the metabolic rate, improve metabolic disorders, enhance cardiopulmonary function, and significantly reduce the risk of obesity, type 2 diabetes, and other metabolic diseases.57 Some studies have demonstrated the anti-inflammatory effects of regular exercise, as well as its ability to modulate the risk of infection and its close relationship with the immune system.
Although research on humans is limited, it has been demonstrated that physical exercise can alter the composition and structure of the gut microbiota. One study observed that levels of Faecalibacterium prausnitzii, Roseburia hominis, and Akkermansia muciniphila increased in active women (who exercised at least 3 h a week) compared to sedentary controls.58 The study, conducted using quantitative PCR analysis, revealed significant differences between the two groups, with health-promoting bacterial species being higher in active women. Additionally, this study showed that physical exercise at the level recommended by the World Health Organization can change the composition of the intestinal microbiota.
Exercise has been shown to regulate the gut microbiota through various mechanisms. These include promoting the secretion of neurotransmitters and hormones, increasing intestinal transit, reducing contact between pathogens and the gastrointestinal mucus layer, and releasing myokines.59
In addition to its beneficial effects, long-term excessive exercise has also been shown to have detrimental effects on bowel function. Excessive exercise can decrease blood flow to the digestive system, potentially leading to bowel dysfunction. Intensive exercise redistributes blood from the splenic circulation to tissues with a higher oxygen demand, such as the brain and heart, which can damage enterocytes and disrupt mucosal homeostasis due to intestinal hypoperfusion.60
Relationship between stress and microbiota
The interaction between the gut-brain axis is bidirectional and involves a complex network that affects gastrointestinal homeostasis as well as emotion, motivation, and higher cognitive functions. The intestinal microbiota is influenced by various factors, including medication use, diet, exercise, and psychological stress. Psychological stress can impact the development of gut bacteria by impairing intestinal motility or promoting the consumption of palatable foods.61 Additionally, stress and depression can alter the composition of gut bacteria through stress hormones, inflammation, and autonomic changes. In turn, gut bacteria secrete metabolites, toxins, and neurohormones that can affect eating behavior and mood. Certain types of bacteria may encourage disordered eating and increase susceptibility to stress. Stress and depression often lead to increased inflammation, which can trigger the growth of harmful bacteria, resulting in dysbiosis and increased intestinal permeability. Psychological stress can significantly impact the brain-gut axis, leading to functional gastrointestinal disorders like irritable bowel syndrome and functional dyspepsia, as well as chronic gastrointestinal disorders such as ulcerative colitis and Crohn’s disease.62
Conclusions
In the contemporary era, a multitude of factors, including lifestyle, industrialization, and the rising food demands of modern society, have collectively contributed to an elevated prevalence of environmental influences on the microbiota. Despite the lack of clarity on the effects of these factors on human health, there is strong evidence linking to certain diseases. In this review, we sought to examine the consequences of the affected microbiota, which forms a large and dynamic ecosystem beginning in the neonatal period and evolving over time. This ecosystem is influenced by numerous environmental factors, which may have either positive or negative consequences for maintaining overall health.
Declarations
Acknowledgement
None.
Funding
The author declared that this study has received no financial support.
Conflict of interest
The authors have no conflict of interest to declare.
Authors’ contributions
FK is the sole author of the manuscript.

 Author information
Author information