Introduction
Lifestyle changes and urbanization make humans more susceptible to various lifestyle diseases. Despite significant resources being invested in disease prevention and treatment, cancer remains one of the leading causes of mortality worldwide. This is due to disease heterogeneity and poor diagnosis. Understanding the ontogeny of different tumor phenotypes is crucial for developing new therapeutic strategies to combat cancer. Since each type of cancer requires a specific treatment strategy, accurate cancer diagnosis is critical for appropriate and effective treatment. Although much advancement in the cancer drug discovery process occurs, newer technologies with higher precision are required.1
Currently, patient-derived xenografts (PDX) and patient-derived tumor cells (PDT) are frequently employed as tumor models for personalized medicine. However, these models have several shortcomings. PDT models often lack cell-type variety, spatial arrangement, and microenvironment, which can negatively impact stem cell cultures.2 PDX models are expensive, have a modest transplantation success rate, and may require prolonged culture time.3 Recent advancements in in vitro cell culture techniques, particularly 3D organoids, have opened new avenues in drug discovery, disease modeling, and personalized therapy.4 Organoids are 3D cell structures derived from diverse stem cells from both healthy and diseased tissues.5 They closely mimic the physiological structure and function of the source tissue, with capabilities for self-renewal and proliferation, making them excellent models for studying cell biology and diseases. Additionally, they maintain genetic stability when multiplied and passaged.6 The wide applications of organoids have led the pharmaceutical research and development industries to adopt 3D culture models in their early drug discovery programs. The major benefit of 3D organoid models is their ability to predict drug candidate efficacy and toxicity, significantly impacting the reduction of attrition rates in drug research and development.7
Patient-derived tumor organoids (PDTOs) have emerged as a novel and effective model for cancer prediction and treatment. Due to their functional and structural resemblance to in vivo tumor tissue, PDTOs have become a promising technology in cancer research, particularly in cancer modeling, personalized therapy, drug screening, and tumor biobanking.8–11 Tumor tissue from a wide range of tumor types has been successfully established in PDTO models.12 The review “Progress in building clinically relevant patient-derived tumor xenograft models for cancer research” highlights the advancements and clinical relevance of PDX models. It describes the advantages of patient-derived orthotopic xenograft models, such as high fidelity to the original tumor, heightened drug sensitivity, and an elevated rate of successful transplantation. However, patient-derived orthotopic xenograft models present significant challenges, including the requirement for advanced surgical techniques and resource-intensive imaging technologies. The review also discusses the development of humanized mouse models and zebrafish models, which provide a human immune environment and offer novel personalized medical models with reduced patient waiting times. This comparison underscores the need for more accessible and less resource-intensive models like PDTOs that retain the genetic, physical, and cellular characteristics of their originating tissue.13 In this review, we will discuss how various types of PDTOs have emerged and how they contribute to the drug development process.
Generation of PDTOs
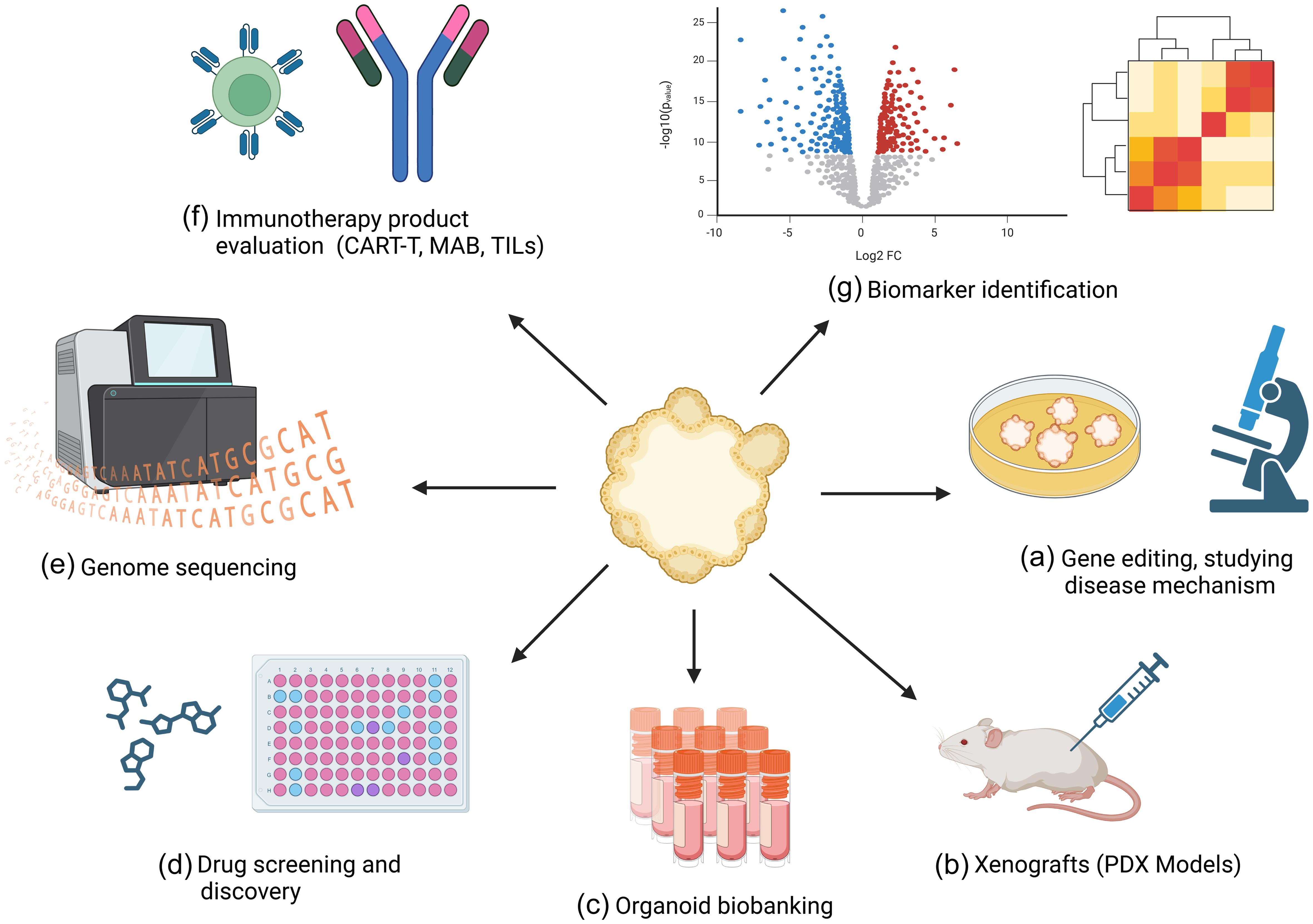
PDTOs are derived from tumor tissues or cancer-specific stem cells. They not only reflect the 3D cellular structure of the original tumor but also retain their functionality, genetics, and intrinsic heterogeneity. Moreover, organoid cultures can be passaged without compromising their genetic stability. The long-term expansion capacity of PDTOs makes them a potential model that may narrow the gap between PDT and PDX models in preclinical research.14 Furthermore, these expanded PDTOs can be cryopreserved and thawed for regeneration, making them suitable for biobanking and compound screening purposes. In general, PDTOs have the potential to move from the bench to the bedside (Fig. 1).15
(a) Utilizing CRISPR/Cas9 and other gene-editing tools to modify genes in tumor organoids to study the genetic underpinnings and mechanisms of cancer. (b) Transplanting patient-derived tumor organoids into immunocompromised mice to create in vivo models that closely mimic human cancer for further research and therapeutic testing. (c) Establishing and maintaining a repository of patient-derived tumor organoids for future research. (d) Testing and evaluating the efficacy and toxicity of various anticancer drugs on patient-derived tumor organoids to identify promising treatments. (e) Performing genomic analysis on tumor organoids to uncover genetic mutations, variations, and potential therapeutic targets specific to an individual’s cancer. (f) Assessing the effectiveness of immunotherapy drugs on tumor organoids, including studying interactions with immune cells to predict patient responses. (g) Identifying molecular biomarkers within tumor organoids that can be used for early diagnosis, prognosis, and tailored treatment strategies for cancer patients.
The utilization of PDTOs could be a significant approach to uncovering the mysteries of cancer. With their ability to replicate the genetic, physical, and cellular characteristics of their originating tissue, PDTOs are suitable for investigating diverse types of tumors. Human pluripotent stem cells or adult somatic stem cells can be used to generate tissue-specific organoids. The process of deriving tissue organoids from stem cells involves growing them in a specialized culture system that mimics the conditions found in the body. This allows the cells to self-organize and differentiate into various cell types.16 Cancer/tumor organoids are generated from an individual’s cancerous tissue and are used to in vitro replicate the biological properties of the tumor, allowing researchers to investigate cancerous cell activity and test potential therapies. Additionally, organoids produced from precancerous lesions can also be used to simulate the early phases of carcinogenesis and investigate the molecular and epigenetic modifications that occur as cells advance toward malignancy. Tumor organoids often grow more slowly than their normal counterparts due to various factors, including mutations affecting cell division and metabolism, as well as differences in the microenvironment. To ensure the growth of pure tumor cells and preserve normal tissues, selective culture conditions are often used. This may involve selecting specific cell types or using specialized media to promote the proliferation of malignant cells while preventing the growth of normal cells.17
The use of basement membrane or matrigel extract as an alternative to extracellular matrix (ECM) is also a critical component of organoid culture. These materials provide the necessary scaffold and biochemical cues for the cells to form three-dimensional (3D) structures and can be augmented with growth factors and other nutrients to support organoid growth and development.18 However, it should be noted that the composition of the ECM can affect organoid growth and behavior, and different types of ECM may be required for the growth of different types of organoids.19 Additionally, organoids require a particular growth medium rich in growth factors and ECM components to expand due to their stem cell origin.20 Finally, if the inner cells become enlarged, they may lose contact with nutrients and die through apoptosis, necrosis, or senescence.21 In their recent work, Kim and colleagues elegantly described both the benefits and drawbacks of human organoids.22
Over the last few years, several protocols have been established for the generation of PDTOs from various tumors, including brain, breast, lung, gall bladder, and liver.23–27 The composition of the media and niche factors is critical to ensuring the dependability and reproducibility of results.27 The various media components used to generate tumor organoids are described below in Table 1.22,24,26–34
Details of specific tissue-specific combinations of growth factors and signaling factors to allow propagation of Culture systems of different Patient derived tumor organoid models
| Supplements | Breast cancer | Lung cancer | Liver cancer | Gall bladder adenoma/carcinoma | Head and neck cancer | Pancreatic cancer | Gastric cancers | Ovarian cancer | Cervical cancer |
|---|---|---|---|---|---|---|---|---|---|
| Base medium | DMEM | Advanced DMEM/F12 | DMEM/F12 | DMEM/F12 | Advanced DMEM/F12 | DMEM/F12 | DMEM/F12 | Advanced DMEM-F12 | Advanced DMEM/F12 |
| Antibiotic/Antimycotic | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| L-Glutamine | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| HEPES | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Primocin/Plasmocin | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Wnt-3a | ✓ | ✓ | ✓/✗ | ✓ | ✓ | ||||
| R-Spondin | ✓ | ✓ | ✓ | ✓/✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Noggin | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| B27 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| N2 | ✓ | ✓ | |||||||
| Nicotinamide | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| N-Acetyl-Cysteine | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Gastrin | ✓ | ✓ | ✓ | ||||||
| A83-01/ SB431542 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| CHIR99021 | ✓ | ||||||||
| Y27632 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| SB202190 | ✓ | ✓ | ✓ | ✓ | |||||
| EGF | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| FGF2 | ✓ | ||||||||
| FGF7 | ✓ | ✓ | |||||||
| FGF10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| HGF | ✓ | ✓ | |||||||
| IGF | ✓ | ||||||||
| Hydrocortisone | ✓ | ✓ | |||||||
| Prostaglandin E2 | ✓/✗ | ✓ | |||||||
| Prostaglandin E3 | ✓ | ||||||||
| β-Estradiol | ✓ | ✓ | ✓/✗ | ||||||
| Transferrin | ✓ | ||||||||
| Forskolin | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Sodium Pyruvate | ✓ | ||||||||
| L-cysteine | ✓ | ||||||||
| FBS | ✓ | ✓ | |||||||
| References | Chen et al.24 | Kim et al.22 | Narayan et al.27 | Yuan et al.26 | Tanaka et al.28; Driehuis et al.29; Perréard et al.30 | Watanabe et al.31 | Song et al.32 | Bi et al. 33 | Lõhmussaar et al.34 |
PDTOs in oncology
PDTOs have revolutionized cancer research by providing a valuable resource for understanding the complex cellular interactions present in tumors. By recapitulating the 3D architecture of tumors in vitro, PDTOs offer a unique opportunity to explore tumor biology and test new therapeutic methods in a more physiologically relevant environment. Organoids have already been utilized to examine a wide range of cancers, involving colorectal, breast, pancreatic, lung, and brain tumors, yielding vital insights into the molecular processes underlying tumor initiation, development, and drug resistance.35,36
One of the major benefits of PDTOs in oncology is their potential to enable personalized medicine. By generating PDTOs, researchers can evaluate the effectiveness of different drugs on a patient’s tumor cells before administering them to the patient, allowing for more targeted and effective treatment.37 Additionally, organoids can represent tumor heterogeneity, which is a significant obstacle in cancer therapy, enabling scientists to examine the behavior of numerous tumor cell types and assess the effectiveness of combination therapies.38
Organoids are currently having a major influence in oncology. Organoid technology has been successfully applied in tumor research, where these 3D structures are often considered true mini-organs.39 In fact, organoids can be envisioned as accelerators for medical research and personalized healthcare, as they represent a tool for investigating human pathophysiology with fewer ethical issues compared to animal models.40 Organoids are a 3D miniature approximation of a heterogeneous organ or tissue and serve as a feasible pathophysiological system.41 Furthermore, they can be maintained in culture for extended periods, grown, and preserved in liquid nitrogen, making them an excellent model for screening purposes.19 Despite their many advantages in oncology, there are challenges associated with their use for personalized medicine and drug screening. For example, tumor organoids can be genetically heterogeneous, which can complicate the identification of effective therapies that target all tumor cell populations.42 Additionally, tumor organoids may not fully replicate the complexities of the tumor microenvironment, which can limit their predictive value for in vivo drug responses. Despite these challenges, the utilization of tumor organoids for drug screening and customized treatment is an exciting field of research with the potential to improve tumor treatment outcomes.
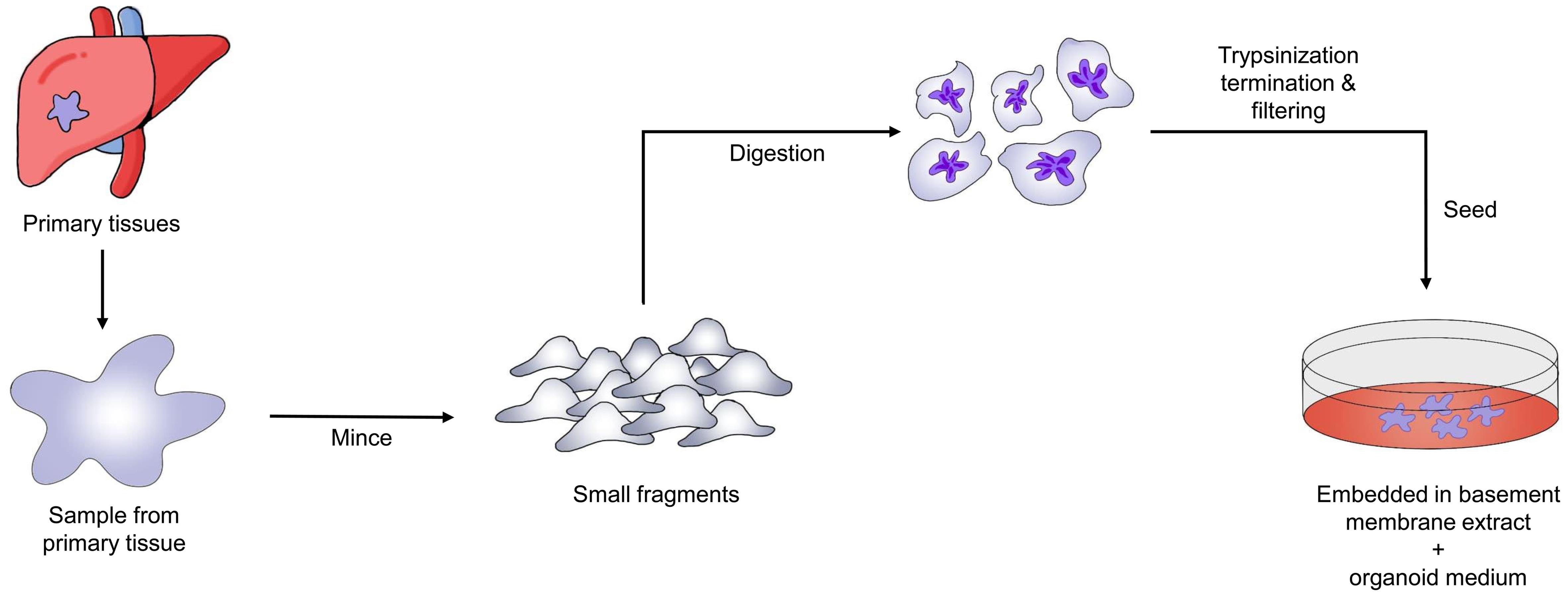
PDTOs from liver tumors
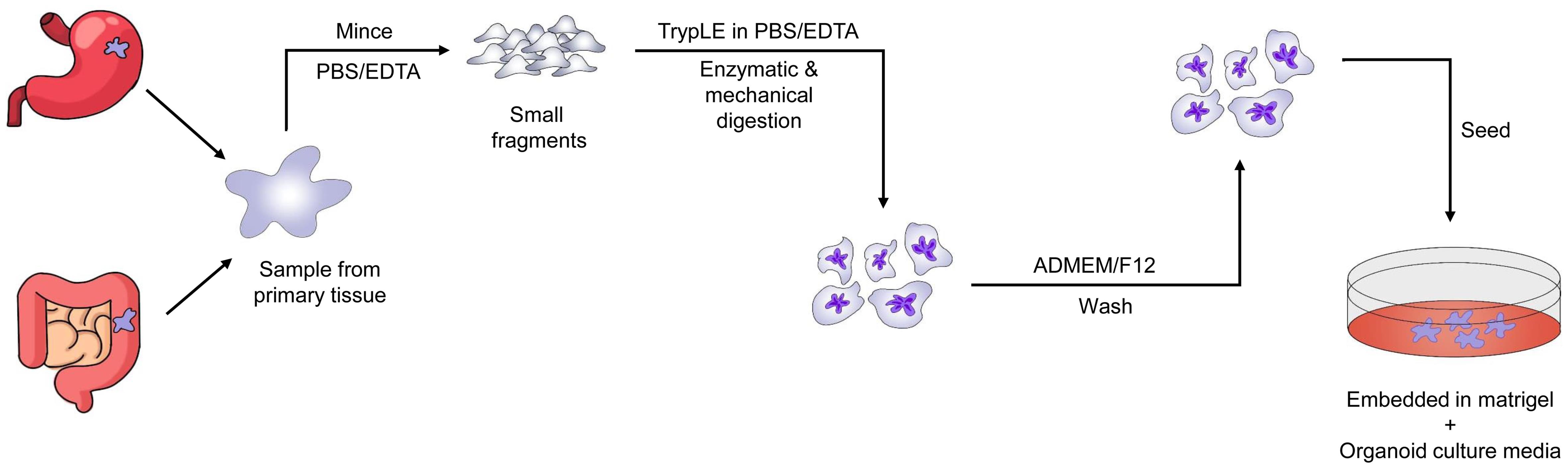
Three forms of liver cancer organoids—hepatocellular carcinoma (HCC), cholangiocarcinoma, and HCC coupled with cholangiocarcinoma—have been generated using standard human tumor organoid culture medium and tumor-specific tissue culture medium.43 Organoids generated from mouse liver malignancies have also been produced and characterized in certain studies.44Figure 2 illustrates the overall process for creating PDTOs from liver cancer. These organoid constructs have the potential to significantly advance liver cancer research, aid in understanding signaling pathways that interact with specific cell types, and serve as tools for personalized therapeutics.45 However, there are no alternative methods to examine the initial stages of liver cancer. Sun et al. used reprogrammed human-induced hepatocytes to generate liver cancer organoids, demonstrating that these organoids may reveal the early stages of cancer.46 Moreover, Artegiani et al. used CRISPR/Cas9 technology to construct human liver cancer organoids, providing a model to understand the genetic causes of tumor growth.47
Chemotherapeutic resistance is common in many types of cancer. Hepatic cancer organoids have proven useful in identifying mechanisms of anticancer drug resistance. Sorafenib, for example, is commonly utilized to treat HCC; nevertheless, the fundamental mechanism of resistance remains unidentified. There is growing evidence that cancer-causing cells contribute significantly to resistance, with Hedgehog signaling and CD44 playing crucial roles in the tumor-initiating properties of HCC cells.48 Wang et al. investigated the functions of CD44 and Hedgehog signaling in HCC and explored the therapeutic effects and resistance to sorafenib.49 Additionally, organoids have been used to demonstrate intra-tumor genetic heterogeneity, which may be a factor in liver cancer treatment failure. Li et al. produced 27 liver cancer organoid cell lines and evaluated them with 129 cancer treatments to assess heterogeneity in primary human hepatic cancer.50 They generated 3,483 cell survival data points, demonstrating the intra- and inter-patient functional variability of liver cancer organoids. One option for treating HCC is liver transplantation. Nonetheless, a significant side effect of liver transplantation is tumor recurrence due to immunosuppression. The potential of mycophenolic acid, an immunosuppressant commonly used after liver grafting to prevent tumor recurrence, has been tested using mouse liver cancer organoids.51 These observations demonstrate the true potential of organoid technology for drug screening and hepatic cancer modeling and suggest its potential for personalized treatment of liver cancer. Li et al. (2019) developed 27 hepatic tumor organoid lines from primary human liver cancer tissue, derived from different tumor types, and tested 129 cancer drugs.52 They found that a subset of nine cancer medications approved by the U.S. Food and Drug Administration showed at least moderate activity in most organoid lines, indicating the potential of this technology for precision medicine. Hui Yang et al. examined a significant challenge in precision medicine for primary liver cancer (PLC), namely inter- and intra-tumor heterogeneity.53 They demonstrated the reliability of a PLC biobank for drug sensitivity screening by assembling 399 tumor organoids from 144 patients. These organoids accurately mimicked the histology and genomic landscape of the parental tumors, and patient responses and in vivo models corroborated these findings. Using an integrative approach, the study analyzed the genetic and transcriptomic features of PLCs, their sensitivity to seven therapeutically relevant drugs, and potential clinical connections. This pharmacogenomic research aimed to improve patient stratification by discovering and validating multi-gene expression patterns that predict drug response. Additionally, the study identified c-Jun, through JNK and β-catenin signaling, as a key player in lenvatinib resistance. A molecule (PKUF-01) was synthesized and tested, combining lenvatinib and veratramine (a c-Jun inhibitor), which showed a strong synergistic effect. The researchers developed predicted biomarker panels, characterized the landscape of PLC heterogeneity, and identified a mechanism for combination therapy resistant to lenvatinib.53
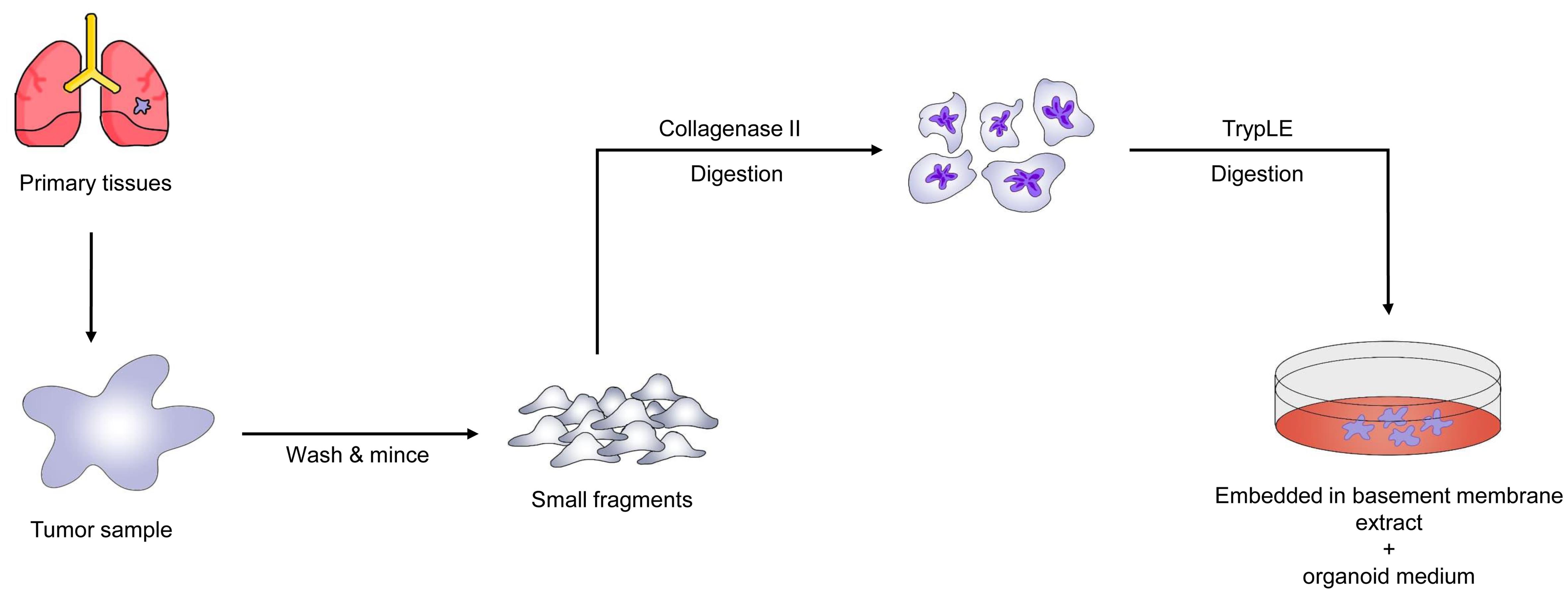
PDTOs from lung diseases
Individuals with lung cancer exhibit significant phenotypic and genotypic heterogeneity, necessitating personalized therapy. We provide in vitro models of lung cancer and normal bronchial mucosa obtained from patient tissue, representing five histological subtypes of lung cancer. Lung cancer organoids retain the genomic alterations of the original tumors while reproducing the tissue architecture of primary lung tumors during long-term in vitro growth. The cellular components of the bronchial mucosa are retained in normative bronchial organoids. EGFR-mutant organoids respond to erlotinib, BRCA2-mutant organoids respond to olaparib, and EGFR-mutant/MET-amplified organoids respond to crizotinib. These medication responses are based on the genetic alterations of the lung cancer organoids. Given the short time span between organoid development and drug testing, our newly created model may be effective for early prediction of patient-specific drug responses through in vitro drug trials. Figure 3 depicts the step-by-step procedure for generating lung cancer PDTOs.51
Recent drug screening of lung cancer organoids (LCOs) showed that sensitivity to drugs was determined by genomic modifications: BRCA2-mutant organoids are sensitive to olaparib, EGFR-mutant organoids are sensitive to erlotinib, and EGFR-mutant/MET-amplified organoids are sensitive to crizotinib. Recently, Hu et al. (2021) developed a mechanical sample processing method for generating LCOs from patient-derived tissues, identifying similar histological and genetic features to those of primary tumors.53,54 The processing also facilitates the generation of LCOs in multi-well plates for immediate drug screening. Preliminary evaluation of chemotherapeutic agents and their combinations using LCOs produced results similar to those from PDX models. These findings support the potential of 3D organoid technology for forecasting in vivo tumor responses to therapeutic agents and evaluating the most effective treatments in customized cancer treatment.55 Qiyue Luan et al. developed spheroids containing NSCLC cells (NCI-H358 or A549) and NSCLC pathological diagnostic observations (PDOs) (F231 or F671) co-cultured with WI-38 fibroblast cells.56 The results demonstrated that the spheroids were of uniform size (with a coefficient of variation less than 30%), healthy (with a vitality level exceeding 80% after one week), and resistant to drugs generated by fibroblasts. The PDOs multiplied and remained viable for more than two weeks (over 81%). Adagrasib, an inhibitor of KRASG12C, reduced cytotoxicity in KRASG12C-mutant spheroids co-cultured with fibroblasts or their supernatant. Tumor model proliferation was sustained by fibroblast supernatant. This platform is ideal for in vitro cancer modeling and treatment efficacy evaluation using patient-derived tissues, as it considers the physical properties, viability, and drug resistance imparted by fibroblast supernatants.56
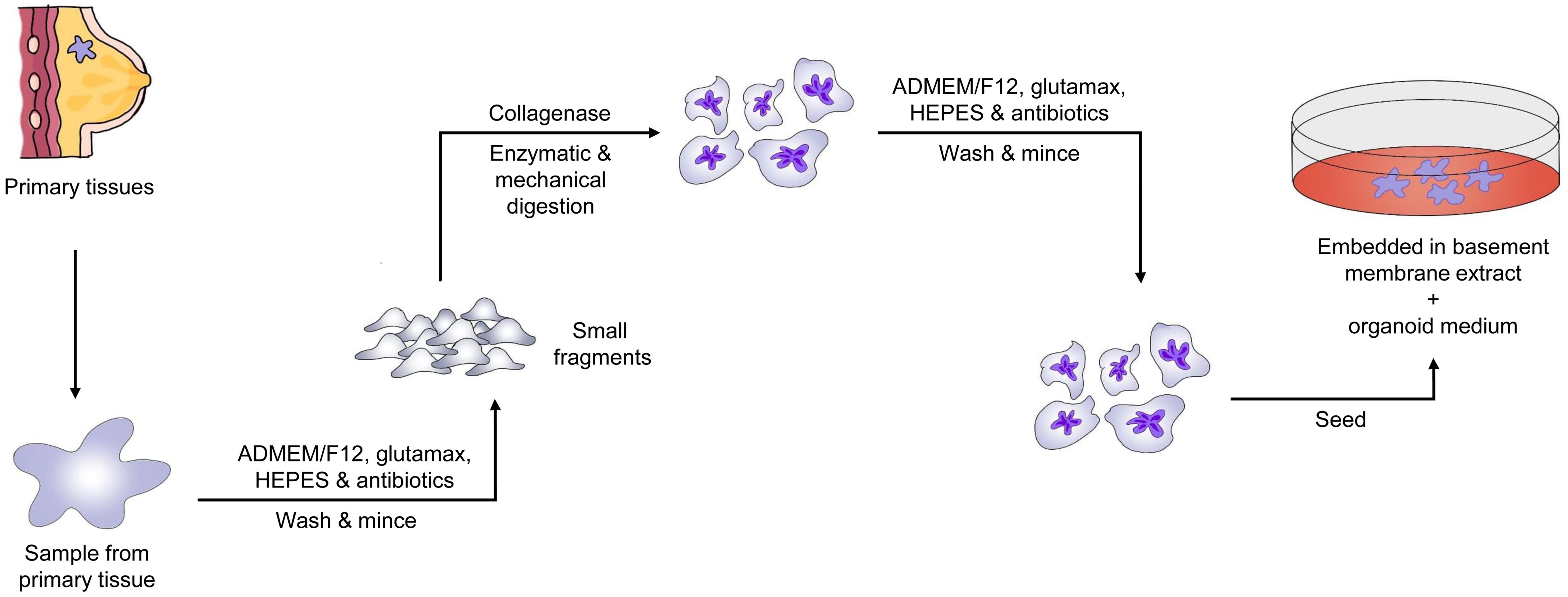
PDTOs from breast cancer
Cancer organoid models facilitate precise treatment and offer a novel approach for drug screening research.57 Tumor tissues cultured on basement membrane extract containing Respond-1, EGF, and Noggin potentially preserve the original cancer tissue’s makeup and self-renewal capabilities.58 This technology has actively influenced drug screening results.59 Drug screening is performed by estimating dosage responses using the area under the curve, which provides the best correlation with patient responses.60 Organoids generated from different tumor specimens have shown similar genetic and molecular characteristics to the initial tumors for several generations, and drug screening results have also been consistent with those from xenografts and clinical tests. For example, PARP inhibitors were effective in organoids with BRCA mutations, while KRAS and BRAF mutant organoids were resistant to olaparib and oxaliplatin, respectively.61,62 A key property of tumor organoids is their ability to be sub-cultured for more than three months or over 30 generations.63 Hence, researchers can establish cancer organoid lines, which not only aid in studying disease-related traits and molecular markers but also enable high-throughput drug screening for precision medicine.64Figure 4 shows the general flowchart for establishing PDTOs from breast cancer, illustrating the steps from patient tissue acquisition to in vitro drug testing. Rowdo, F.P.M. et al. examined the efficacy of poly (ADP-ribose) polymerase inhibitors (PARPi) in treating BRCA1/2 mutations and their prospective advantages for other variations.65 They found that TP53 gene mutations are more common in tumors than BRCA1/2 gene changes. Mutant p53 (mtp53) proteins were observed to interact strongly with replicating DNA and PARP proteins in two-dimensional (2D) breast cancer cell lines. The combination of temozolomide and the PARPi talazoparib effectively killed 2D breast cancer cell lines with mutant p53. PDTOs from breast and lung cancer patients were tested for sensitivity to temozolomide and talazoparib. The two drugs synergistically destroyed cancer cells in PDTOs with mutant p53 but not in those with wild-type p53. Compared to organoids expressing wild-type p53, talazoparib and temozolomide caused more DNA double-strand breaks in mtp53-expressing organoids, as evidenced by increased γ-H2AX protein levels. Furthermore, breast cancer tissue microarrays revealed a link between stable p53 and high PARP1 expression in certain subcategories, indicating PARP inhibitor-sensitive subclasses. These findings suggest that mtp53 may predict the response to PARPi talazoparib and temozolomide.65
PDTOs from gastro-intestinal tract
Many researchers have started using tumor organoids to study tumor organization, development, identify molecular markers, and conduct drug screening. Figure 5 outlines the comprehensive workflow for establishing PDTOs from gastro-intestinal cancers. In a study, gastric cancer organoids (GCOs) were developed from 34 patients, including those with normal, dysplastic, cancerous tissues, and lymph nodes, and performed whole exome and transcriptomic sequencing. These GCOs exhibited similar morphology, transcriptomic, and genomic profiles to in vivo tumors, even after several generations of sub-culturing and passaging. Extensive drug screening revealed sensitivity to drugs such as 5-fluorouracil, cisplastin, napabucasin, abemaciclib, and the Ataxia Telangiectasia and Rad3 related (ATR) inhibitor VE-822. The responses were consistent across different generations of GCOs.66 Similarly, Vlachogiannis et al. (2018) conducted a 3D screening assay over two weeks using a library of 55 drugs currently in phase I-III clinical trials or in clinical practice. ERBB2-amplified organoids exhibited a strong response to lapatinib, while organoids without ERBB2 amplification showed no response. Organoids with a BRAF V600E mutation responded well to vemurafenib but failed to induce apoptosis, unlike those without the BRAF mutation. Paclitaxel response was also similar; organoids derived from patients with a good prognosis had a 4-fold reduced IC50, while resistant organoids showed a dose response similar to patients with refractory paclitaxel.67
PDTOs from colorectal cancer
Costales-Carrera et al. studied the effect of plocabulin, a novel microtubule-disrupting antitumor agent, on colorectal cancer (CRC) organoids derived from three CRC patients. They observed that the cytotoxicity of plocabulin was greater than that of the approved irinotecan analogue 7-ethyl-10-hydroxy-campothecin in various passages. Additionally, plocabulin had a prolonged effect even in washout experiments.68 Buzzelli et al. and Ganesh et al. developed CRC and rectal organoids, respectively, and found that the drug screening results of these tumor organoids were similar to the clinical responses of the patients.69,70 Yao et al. developed rectal cancer organoids from patients enrolled in neoadjuvant chemoradiation therapy. The therapy responses of the patients were highly matched with those of the rectal cancer organoids.71 They found that organoids sensitive to a particular therapy could predict patient response to that therapy. Another study indicated that organoids derived from patients with a positive diagnosis were responsive to at least one chemotherapy treatment. In contrast, organoids from poor-prognosis patients showed resistance to all chemotherapeutic drugs.72 CRC organoids also retained genetic information regarding mutations and copy number changes.73 Drug screening with GDC0941, obatoclax mesylate, and trametinib showed consistent results across different time intervals. Screening of 2,427 compounds processed through machine learning in CRC organoids revealed varying sensitivities, highlighting the utility of organoid screening in precision medicine.74 Schutte et al. integrated genomic data from different CRCs and identified biomarkers for EGFR inhibitors through drug screening in PDX and organoid models.75 Tashiro et al. screened cetuximab, an anti-EGFR antibody, on KRAS-wild-type and KRAS-mutant CRC organoids and observed no response in the mutant organoids.76 These results were reflected in vivo, indicating the usefulness of the 3D organoid platform for understanding diverse mechanisms of drug response. Kong et al. recently published a machine learning system to uncover resilient therapeutic indicators using genomic data from CRC organoids.77 These biomarkers accurately predicted responses to 5-fluorouracil and cisplastin in CRC and bladder cancer patients. The biomarkers were further confirmed with transcriptomic data from drug-resistant and sensitive isogenic lines. Pauli et al. exploited high-throughput medication screening to uncover effective treatment options by integrating genetic data.38 Research identified effective drugs and combinations for two colon cancers and two uterine cancers, which were further validated by organoid cultures, suggesting the use of organoid cultures in precision medicine.78 Iyer et al. tested the cytotoxic anti-tumor effects of high-dose, short-term (HDST) tyrosine kinase inhibitors (TKIs) on five PDTOs.79 Sunitinib, cediranib, and osimertinib were chosen based on their pharmacokinetics and physicochemical properties. Clinically verified LC/MS-MS was used to measure intra-tumoroid TKI levels. Cell death was assessed through enzyme activity assays, immunofluorescent labeling, and western blotting. Most PDTOs responded to sunitinib and cediranib, while all PDTOs responded to osimertinib. Additionally, HDST osimertinib was found to suppress organoid growth, with increased sensitivity and intra-tumoroid TKI concentrations. HDST osimertinib also triggered apoptosis in the PDTOs.79.
PDTOs from bladder cancer
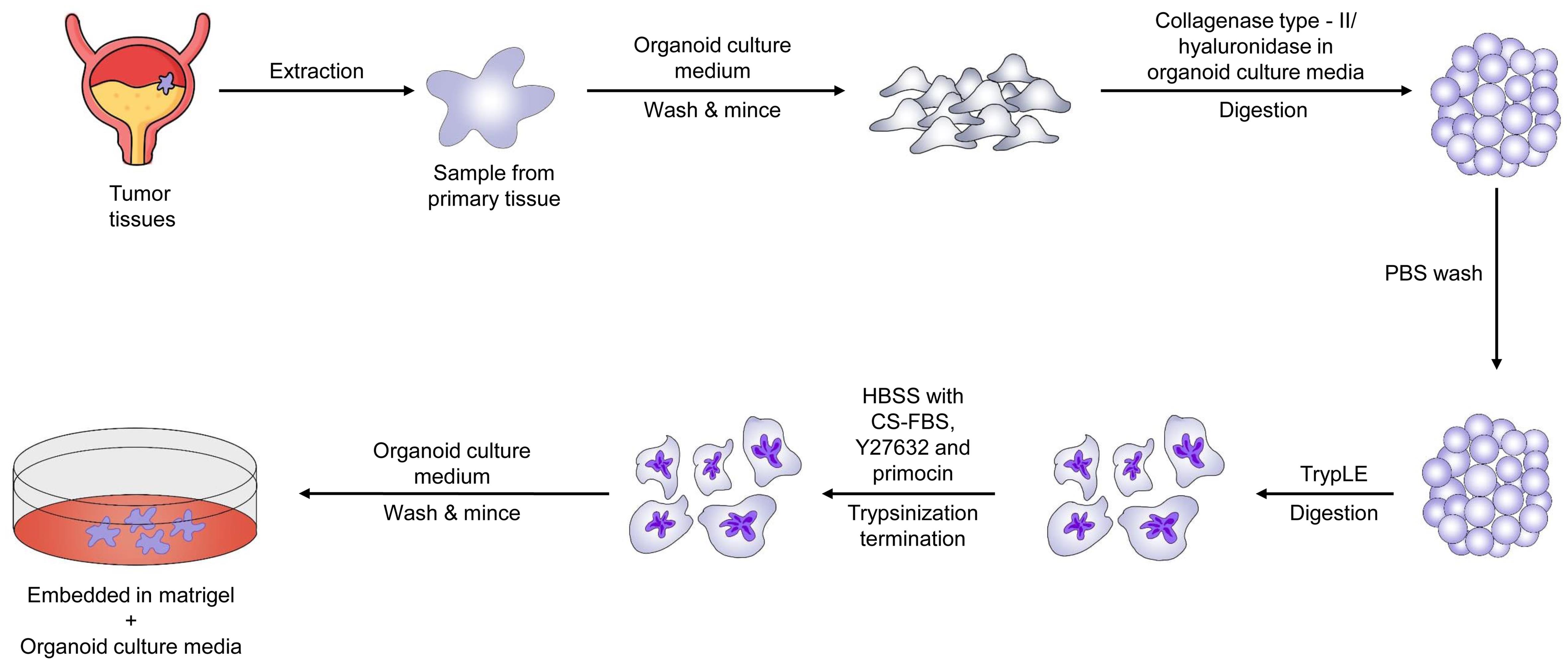
In a study published by Lee et al. in 2018, a biobank of bladder tumor organoids was established from patient tumors.80 The organoids were found to preserve the tumor histopathology and genetic mutations of the original tumors, even after multiple passages during culture. This makes bladder cancer organoids a potentially valuable tool for researching bladder malignancy physiology and evaluating new treatments.80Figure 6 shows the general flowchart for the establishment of PDTOs from bladder cancer. Viergever et al. demonstrated that urine-derived bladder cancer organoids (urinoids) exhibit bladder tumor-like histology.81 Urinoids shared 92.56% of SNPs and 91.54% of insertions and deletions with the original tumors of Patient 4. Similar to tissue-derived organoids, urinoids responded to bladder cancer therapy. Genetic analysis of longitudinally produced tumoroids and urinoids from a patient undergoing systemic immunotherapy suggested potential second-line treatment options. Therapy adaptation was successful in the urinoid setting.81 Consequently, organoids have the potential to enhance precision medicine in bladder cancer by serving as a non-invasive tool for studying tumor development, evaluating treatment responses over time, and adapting therapy accordingly.
PDTOs from pancreatic cancer
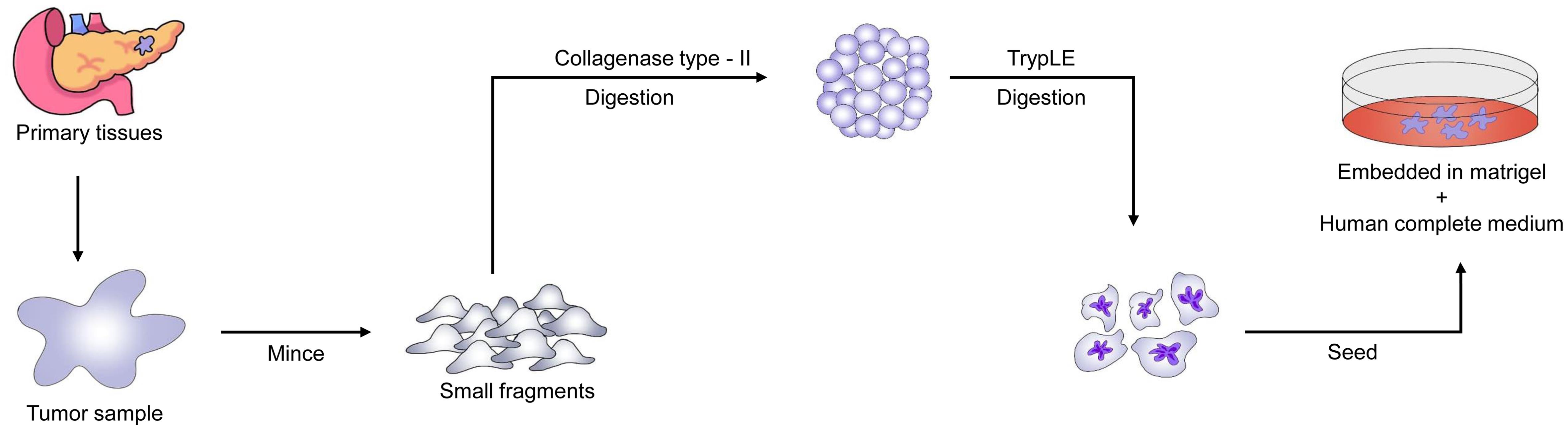
Pancreatic cancer organoids produced from high-prognosis patients were susceptible to at least one chemotherapy treatment. In contrast, organoids developed from poor-prognosis patients showed resistance to all chemotherapeutic drugs. Figure 7 presents a detailed schematic of the process to establish PDTOs from pancreatic cancer tissues. Choi et al. studied the response of gemcitabine in pancreatic ductal adenocarcinoma patient-derived organoids and found results similar to the patients’ clinical outcomes and in vivo PDX.78 Similar studies also observed that drug response assays in tumor organoids were correlated with patient data and PDX models.82–84 These findings highlight organoids’ potential as significant tools for personalized treatment and drug discovery in pancreatic cancer.85,86 Sanjit Roy et al. examined pancreatic tumor organoids in mice to identify pancreatic cancer stem cells (CSCs) using OCT4 and SOX2 markers.87 The pancreatic tumor organoids were treated with four or eight Gy of radiation, 10 µM 5-FU, and 100 µM 3-Bromopyruvate, either alone or in combination with radiation therapy. Results showed that pancreatic cancer organoids treated with four and eight Gy of radiation expressed higher levels of OCT4 and SOX2. Treatment with 5-FU reduced these expressions. While radiation elevated CSC markers, 5-FU chemotherapy lowered them depending on the treatment dose. The combination of 5-FU and radiation inhibited CSC regeneration by suppressing the synthesis of SOX2 and OCT4 in cancer organoids. Compared to normal pancreatic tissue, human pancreatic tumor tissues exhibited abnormal levels of OCT4 and SOX2, indicating their role in cancer development and treatment resistance. Furthermore, the combination of 5-FU and radiation therapy disrupted the β-catenin pathway in pancreatic cancer organoids, thereby increasing treatment sensitivity and promoting cell death.87
PDTOs from ovarian cancer
The TP53 mutation and severe chromosomal instability characterize high-grade serous ovarian cancer.88 Drug profiling of organoids derived from ovarian carcinoma patients with characterized p53 mutations revealed that cisplastin sensitivities are consistent with patient responses. These responses were similar when the treatment was administered at different time intervals. In another study, organoids developed from high-grade serous ovarian cancer were used to study deficiencies in homologous recombination and replication fork protection.89 De Witte et al. generated 36 patient-derived, whole-genome-characterized ovarian cancer organoids and screened them for drug response.90 They observed that the organoids displayed both inter- and intra-patient variability in chemotherapy drug responses. Whole genome analysis suggested that the response might be due to genetic aberrations. They proposed that generating organoids from various tumor sites can enhance the understanding of the genetics of the disease and drug responses, leading to better decision-making during treatment. Organoid lines developed from 32 ovarian cancer patients, utilized in screening standard chemotherapeutic drugs, revealed both sensitive and resistant drugs for particular individuals.91 Jabs et al. tested 22 single drugs or combinations in 10 ovarian cancer organoids and found diverse responses compared to monolayer cultures.92 Besides, the genomic alterations caused by drug responses were similar in both patients and organoid cultures, indicating the practical advantages of 3D organoid cultures. An organoid library created from different endometrial cancer patients, after sub-culturing for over eight months, showed the mutational topography of the initial tumors and patient-specific drug responses.93 Similar results were observed when YM155, a survivin inhibitor, was screened; non-endometrioid adenocarcinomas showed sensitivity while endometrioid adenocarcinomas showed resistance to the drug.94 Chen et al. observed that among the 26 PDOs examined, 15 were associated with ovarian cancer, 11 with endometrial cancer, and two with cervical cancer.95 These PDOs effectively preserved the histological characteristics of the original tumors. Interestingly, even among patients with the same histological type, there was variability in medication sensitivity detected between individuals. Furthermore, individual variations in responses to prescribed medications were noted during in vitro drug testing of eleven patients with ovarian cancer categorized into PDOs. In four patients categorized as platinum-sensitive, platinum-resistant, or platinum-refractory, a significant correlation was observed between clinical outcomes and pharmacological responses in PDOs.95
PDTOs from head and neck cancer
Squamous cell carcinoma of the head and neck (HNSCC) organoids were successfully established, showing identical histological characteristics as well as stem cell, epithelial, and mesenchymal markers compared to the parental tumors. The sensitivity of these organoids to cisplastin and docetaxel was also correlated with responses observed in 2D cell lines and in vivo.28 Similar studies performed by Driehuis et al. observed differential drug responses in organoids to cisplatin, carboplatin, cetuximab, and radiotherapy in vitro. They also noted sensitivity to targeted drugs not yet approved for cancer treatment, highlighting a personalized approach to the management of HNSCC.29
Pauli et al. employed high-throughput drug screening to identify effective treatment options by integrating genetic data.38 They discovered effective drugs and combinations for two colon malignancies and two uterine malignancies, which were further validated by organoid cultures, suggesting the potential of organoid cultures in precision medicine. Organoids provide a reliable model system for assessing tumor growth and treatment response in the context of precision cancer medicine.
To validate the similarity between PDTOs and their initial tumors, Marion Perréard et al. conducted a study involving histological and immunohistochemical characterizations.30 They utilized live-cell imaging and viability assays to assess the response of PDTOs to various treatments, including chemotherapy, radiation, and novel therapeutics. To improve the success rate of PDTO establishment, which has now exceeded fifty percent, the researchers optimized the culture conditions. Twenty-one PDTO lines were produced, demonstrating histological characteristics similar to those of the initial tumors. Immunohistochemistry revealed that tumor markers such as p53, p40, p63, and p16 were expressed in both tumors and paired PDTOs in a comparable manner. Fifteen PDTOs underwent functional assays to investigate their reactions to various treatments, including cisplatin, olaparib, and X-rays. Different models exhibited varying degrees of response to these therapies. PDTOs derived from patients who responded well to therapy showed high sensitivity to the treatments, while those from patients who did not respond well demonstrated lower sensitivity. Notably, two PDTO lines generated from HPV+ oropharyngeal tumors exhibited very high sensitivity to cisplatin, consistent with the patients’ profiles and responses. These findings suggest the feasibility of generating PDTOs from head and neck squamous cell carcinoma and conducting functional assays to evaluate patients’ reactions to various therapies. Initial studies investigating the association between PDTOs and patient responses have shown promising results, prompting further investigation of additional patients in the future. This will help demonstrate the predictive value of PDTOs from HNSCC, which is essential for designing predictive functional assays that align with clinical settings.30
Limitations and future prospective
An inherent disadvantage of PDTOs, like all organoids, is that they cannot replicate whole-organ interactions, such as tumor-stroma, tumor-microenvironment (including microbiome), tumor-endothelium, and tumor-multi-organ interactions. In fact, PDTOs lack the architecture of a complete organ, including the surrounding normal tissue.96 They are devoid of a functioning immune system and a whole-body axis, including all intercellular networks. Furthermore, PDTOs lack vasculature, which means they cannot grow too large without encountering issues related to efficient nutrient transport, hypoxia, and necrosis in the interior parts.97 Finally, the organoid creation process involves an inherent cell-selection process that should be considered at all times.98
Despite their many advantages, there are still several challenges associated with using organoids in oncology. For example, organoids do not contain immune cells, which have a significant impact on tumor growth and response to therapy. To address this limitation, researchers are working on incorporating immune cells into organoid models.99 Additionally, organoids lack a functional blood vessel network, which limits their ability to model tumor angiogenesis and metastasis. Researchers are also developing vascularized organoid models.100
Recent organoid research is attempting to address these limitations using both traditional and innovative technologies. PDTOs can be genetically modified, co-cultured with immune and stromal cells, and micro-conjugated with microorganisms.101–103 Furthermore, PDTOs can be transplanted into humanized mice to produce PDX, which provide body axis complexity.31,104,105 The development of new, groundbreaking 3D bio-printing and bio-fabrication materials, facilitated by bioengineering, holds promise for overcoming these challenges. The development of organoid-on-a-chip systems could lead to PDTOs with improved interconnectivity with other cell types, closely mimicking the body axis and reconstructing the microfluidics of the entire organism.23,106–108
Conclusions
Organoids have had a profound impact on oncology by providing a powerful tool for studying tumor biology and testing new therapeutic approaches. While there are still challenges associated with using organoids in oncology, ongoing research is addressing these limitations. Organoids are likely to become increasingly significant in the fields of personalized medicine and tumor research in the years to come.
Declarations
Acknowledgement
None.
Funding
None.
Conflict of interest
None.
Authors’ contributions
Review conceptualization, project supervision, manuscript review and editing (SM), data curation, and manuscript drafting (BS).

 Author information
Author information