Introduction
Autism spectrum disorders (ASD) encompass a wide range of neurodevelopmental conditions characterized by heterogeneous cognitive, behavioral, and communication impairments that typically manifest in early childhood.1 Common features of autism include impaired communication skills, social withdrawal, and a repetitive or restrictive pattern of behavior, interests, and activities. These diagnostic traits usually appear early in the development of the patient. Additional behaviors often reported include picky eating habits, increased aggression, and anxiety.2 Regressive autism or late-onset autism refers to a subgroup of patients with initially normal development, who gradually lose acquired communication or social interaction skills.3 The diverse phenotypic manifestations of ASD suggest that multiple factors contribute to its etiology. While both genetic and environmental influences are implicated, most cases of ASD are isolated, with the precise cause remaining unknown. To date, over 100 genes have been identified as putatively associated with ASD.4 However, many genetic variants are linked with heterogeneous phenotypes, making it difficult to identify the molecular mechanisms responsible for specific impairments.5 A notable characteristic of individuals with ASD is abnormal eating habits, which may lead to vitamin, mineral, and fatty acid deficiencies.6 Additionally, gastrointestinal (GI) disorders are more commonly observed in autistic children,7 with symptoms such as constipation, diarrhea, or bloating reported at higher rates compared to neurotypical children.8 These findings suggest a relationship between the GI tract and the brain in individuals with ASD. Gut microbiota is thought to play a key role in this relationship by influencing the central nervous system through various mechanisms.9 Further evidence supporting the importance of gut microbiota in ASD is the observed reduction in behavioral and GI symptoms when autistic children are treated with probiotics and/or antibiotics.10 However, the relationships among the altered eating habits of these children, more frequent GI disorders, changes in the gut microbiome, and their correlation with the severity of autistic manifestations are not yet fully understood.
This review aims to highlight the main findings regarding changes in the gut microbiome in ASD, the factors that influence it, and its potential role in the pathogenesis of ASD. A better understanding of these mechanisms and how to modulate them may lead to new approaches for preventing and treating these conditions.
Factors influencing the microbial composition in children with autism
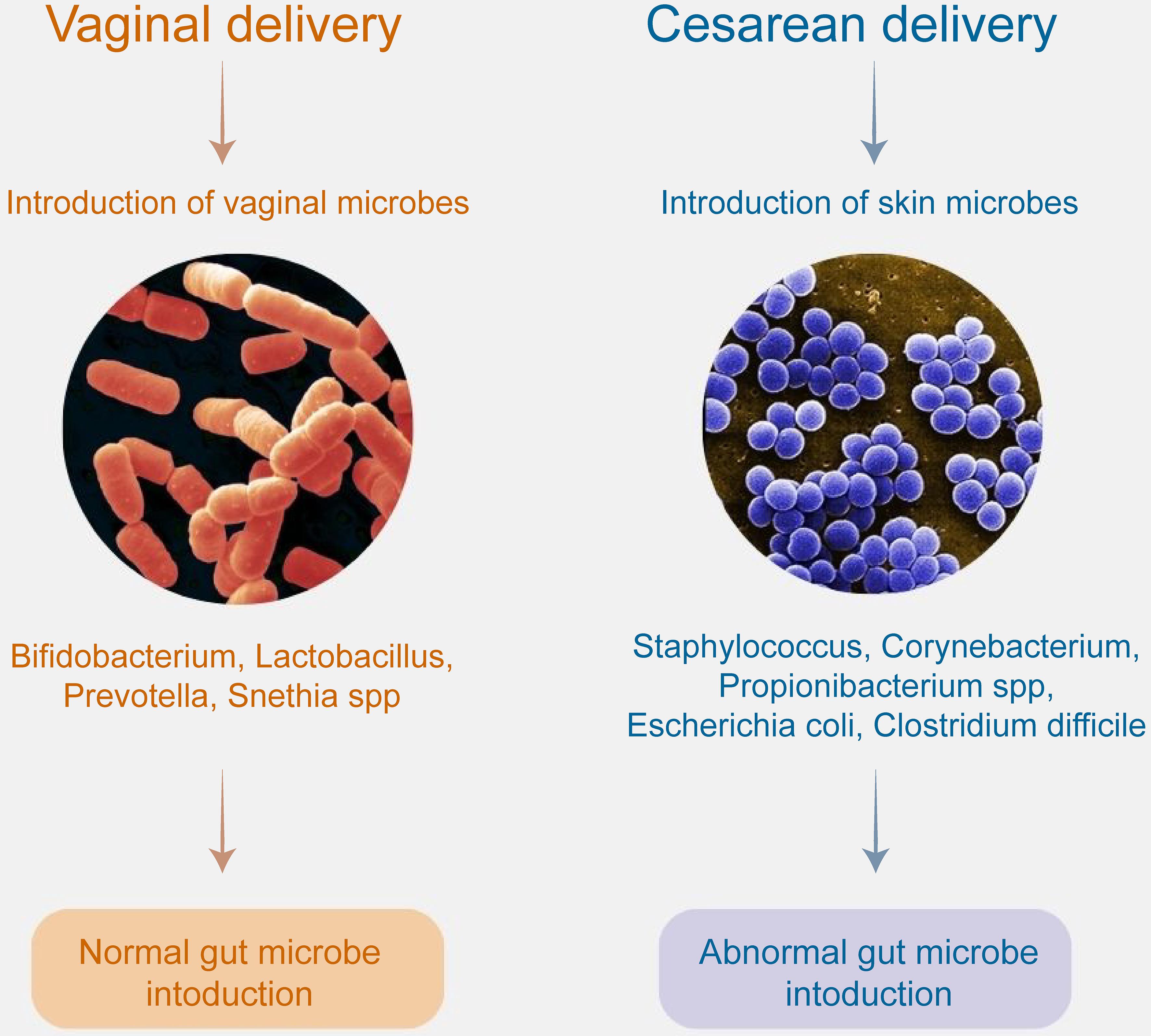
According to a meta-analysis, children born by cesarean section have a 23% higher risk of developing ASD compared to those born via vaginal delivery.11 Another multinational study, which included 5 million births from Norway, Sweden, Denmark, Finland, and Western Australia, similarly reported a higher risk of ASD in children born by cesarean section. However, this study did not establish a relationship between the gestational age at the time of delivery and the risk of developing ASD.12 Premature birth, mode of delivery, and breastfeeding influence the microbial composition of the newborn’s gut (Fig. 1). Babies born vaginally and breastfed tend to have healthier microbiota, with higher levels of beneficial bacteria such as Bifidobacterium, and lower levels of pathogenic bacteria, including Clostridium difficile and Escherichia coli.13 Vaginally delivered infants have a microbiota resembling their mother’s vaginal microbiota, dominated by Lactobacillus, Prevotella and Snethia spp. In contrast, infants born by cesarean section exhibit a microbiota that resembles the mother’s skin, with the dominance of Staphylococcus, Corynebacterium, Propionibacterium spp., Escherichia coli, and Clostridium difficile.14
Regardless of the mode of delivery, evidence suggests that changes in the vaginal microbiome during pregnancy may contribute to the development of ASD in offspring.15 One proposed mechanism for this interaction is maternal immune activation, which is thought to affect fetal neurodevelopment. Maternal immune activation refers to the activation of the maternal immune system during pregnancy, leading to the production of cytokines and other proinflammatory molecules that can cross the placenta and influence fetal brain development. Dysbiosis, or an imbalance in the microbiome, may trigger both local and systemic inflammation or predispose the mother to infections, further stimulating the immune system.16 In vaginally delivered children, the composition of the maternal vaginal microbiome is crucial, as it provides the newborn with its first colonizers. Vaginal dysbiosis, such as a reduction in Lactobacillus species, may negatively affect early neurodevelopment. Factors such as maternal stress or antibiotic use during pregnancy can disrupt the maternal vaginal microbiome, leading to dysbiosis and potentially affecting fetal brain development.17
Another factor contributing to microbial dysbiosis is the early and excessive use of antibiotics. This can affect the gut-brain axis by causing epigenetic modifications that impact genes associated with ASD, potentially contributing to the development of the disorder.18 Madore et al. also observed a higher risk of ASD in children who were born prematurely, with very low birth weight, by cesarean section, or who experienced prolonged hospitalization. Additionally, children treated with antibiotics for extended periods or whose mothers had infections during pregnancy were found to have an increased risk of developing ASD.19
GI disorders in children with autism
In addition to dysbiosis, GI symptoms are four times more prevalent in children with ASD compared to the general population.20 These children experience a wide range of GI symptoms, including constipation, diarrhea, bloating, abdominal pain, reflux, vomiting, meteorism and foul-smelling stools. Food allergies are also more common in this population. Children with autism are less likely to consume foods high in glutamic acid, serine, choline, phenylalanine, leucine, tyrosine, valine, and histidine, all of which play a role in neurotransmitter biosynthesis.21
A study comparing 230 preschool children revealed that those with autism suffered significantly more from GI problems than healthy controls did.20 ASD patients with GI symptoms were reported to experience more anxiety and other somatic complaints than those without GI symptoms. In addition, GI disorders in children with autism are associated with an increase in tantrums, aggressive behavior, and sleep disturbances, which further exacerbate behavioral symptoms compared to autistic individuals without GI symptoms.22 A review by Ding et al. suggested that behaviors such as aggression, self-injury, or sleep disturbances in children with autism may be manifestations of abdominal discomfort.23
According to Naviaux, children with autism exhibit altered metabolism and decreased absorption of disaccharides in the gut epithelium.24 The sodium-dependent glucose co-transporter (SGLT1) and glucose transporter 2 (GLUT2) actively transport glucose, galactose, and fructose across the lumen and basolateral membranes of enterocytes. Children with autism have been reported to have significantly reduced mRNA levels of both hexose transporters (SGLT1 and GLUT2) in the ileum. As a result of malabsorption in the small intestine, increased amounts of mono- and disaccharides enter the large intestine, promoting the growth of bacteria that ferment these sugars, thereby altering the microbial composition in the GI tract. The presence of excess sugars in the colon can lead to osmotic diarrhea or serve as substrates for gas production, further contributing to GI distress.25
Changes in the microbiome in children with autism
Significantly increased bacterial species in children with autism include Akkermansia muciniphila, Anaerofilum, Barnesiella intestinihominis, Clostridium spp., Dorea spp., Enterobacteriaceae, Roseburia spp., Parasutterella excrementihominis, and Turicibacter spp. At the same time, the abundances of Bifidobacterium, Fusobacterium, Oscillospira, Sporobacter, Streptococcus, and Subdoligranulum are significantly reduced. The genera Collinsella spp. are also underrepresented, except for Collinsella aerofaciens, as well as Enterococcus spp., Lactobacillus, Lactococcus, and Staphylococcus.3 Kang et al.26 compared the gut flora of twenty autistic children with GI problems to that of twenty neurotypical children. They found significantly lower bacterial diversity in children with autism, which correlated with the severity of their GI symptoms.
A common observation is that Bifidobacterium spp. are less prevalent in the intestines of children with autism, whereas Clostridia spp. are more abundant. A simulation by Weston et al. revealed a two-way relationship between the anti-inflammatory genera Bifidobacterium and the pro-inflammatory Clostridia and Desulfovibrio. Bifidobacterium is inhibited by lysozyme and the growth of Desulfovibrio.27 To some extent, Desulfovibrio thrives on metabolites produced by Bifidobacterium. On the other hand, Clostridia growth is suppressed by both lysozyme and the presence of Bifidobacterium. The authors suggest that an increase in Clostridia in the gut, especially when Bifidobacterium levels are low, may be linked to a higher risk of developing ASD.
Other microorganisms reported to be relevant in children with autism include Candida spp. Several studies have shown a higher amount of Candida spp., particularly Candida albicans, in autistic children.28,29 Altered microbial diversity in autistic populations may favor fungal growth. For example, Lactobacillus spp. stimulate the immune system to produce IL-22. IL-17 and IL-22 together inhibit the overgrowth of Candida spp. Once Candida spp. become established in the gut, they prevent recolonization by commensal microbes. In a dysbiotic environment—often observed in the autistic population—Candida proliferates and produces ammonia and toxins, which are reported to exacerbate autistic behavior. Candida spp. may also cause malabsorption of minerals and carbohydrates, potentially playing a role in the pathophysiology of ASD.
The possible impact of some changes in microbiome composition is represented in Table 1.3,13,30–37
Gut microbiome changes in children with autistic spectrum disorders (ASD) and the possible impact.
| Change | Genus/Species | Possible impact | References |
|---|---|---|---|
| Increased | Clostridium spp | Excess propionic acid production; Toxin production; Altered tryptophan metabolism leading to reduced serotonin levels; Dysregulation of dopamine signaling; Disruption in the secretion of Gamma-Aminobutyric Acid (GABA) and glutamate; Elevated histamine level; Disruption of blood-brain barrier | De Angelis (2013)3; Abdelli (2019)30 |
| Enterobacteriaceae | Increased lipopolysaccharides (LPS) production leading to inflammation; Decreased serotonin production | Xu (2019)31 | |
| Desulfovibrio spp | Increased hydrogen sulfide production which disrupts gut barrier function, alters gut motility and may lead to gut inflammation | Finegold (2011)32 | |
| Candida spp | Stimulation of immune system leading to inflammation; Production of toxic metabolites such as ethanol and acetaldehyde which may increase neuroinflammation | Herman (2022)33 | |
| Decreased | Bifidobacterium spp | Lower levels of neurotransmitters GABA and serotonin; Impaired carbohydrate fermentation; Reduced Treg activity; Deficiency of B-vitamins | Niu (2019)34; Ha (2021)35; Srikantha (2019)13 |
| Bacteroides spp | Lower levels of short chain fatty acids (SCFA); Altered immune function; Reduced synthesis of neurotransmitters – serotonin; Impaired carbohydrate metabolism | Niu (2019)34; Ha (2021)35; Xu (2019)31 | |
| Lactobacillus spp | Immune system dysregulation; Decreased Gamma-Aminobutyric Acid (GABA); Reduced serotonin levels; Reduced SCFA | Taniya (2022)36 | |
| Akkermansia muciniphila | Disruption of mucus layer production which can lead to increased gut permeability and gut inflammation | Wang (2011)37 |
Possible mechanisms and the gut-brain axis
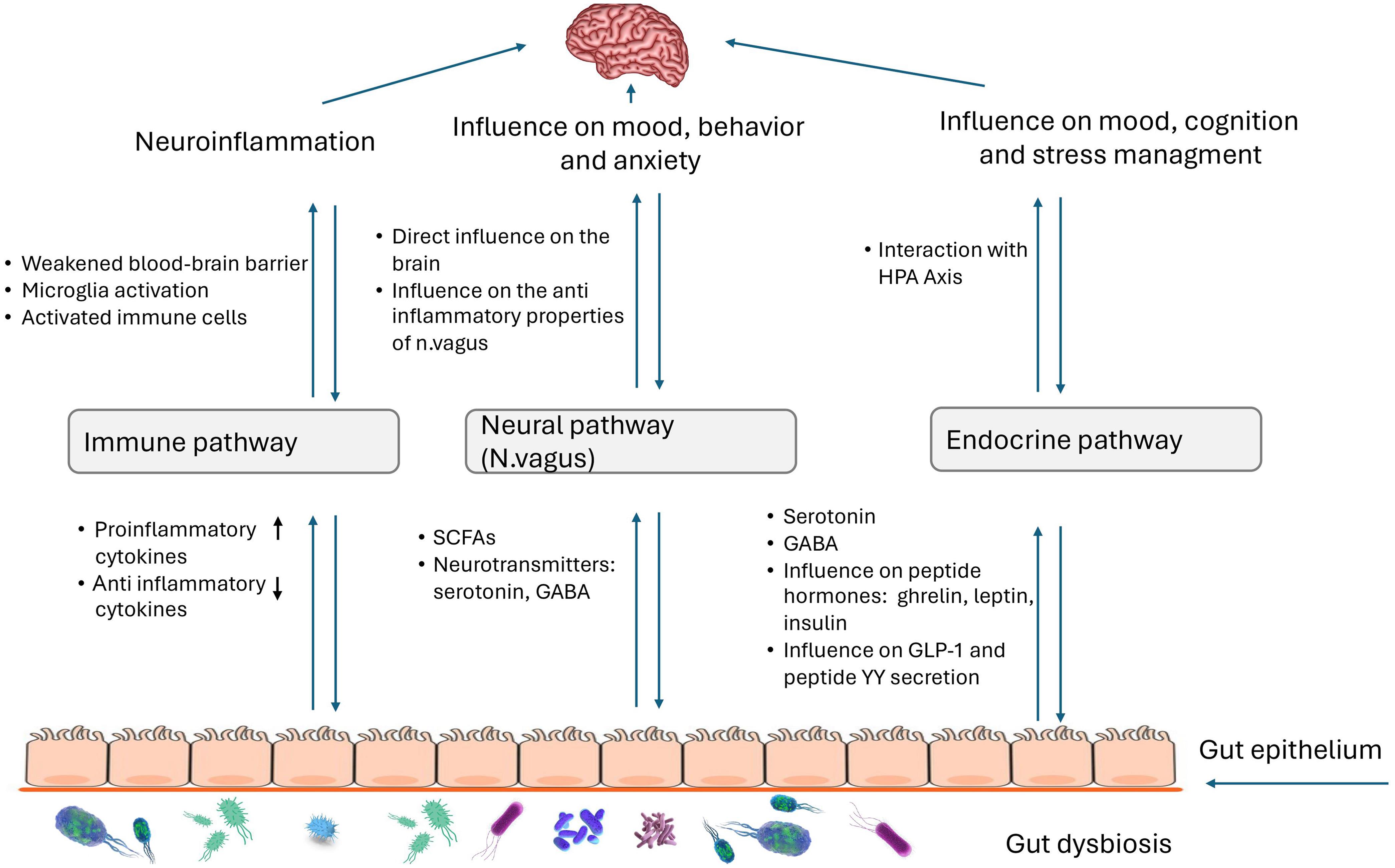
The gut-brain axis is defined as the complex bidirectional communication system between the central nervous system and the enteric nervous system.38 Research is being conducted on the gut-brain axis (GBA) and its relationship with various neurological disorders, including autism. Over the past decade, studies on GBA-modulating factors have revealed the central role of the gut microbiome—the trillions of microbes that colonize the gut—in regulating neuroimmune networks, modifying neural networks, and communicating directly with the brain.39 Alterations in the gut microbiome and the subsequent dysregulation of the GBA are thought to contribute to the pathogenesis of neurodevelopmental disorders, including autism. However, the precise mechanisms and the extent to which the microbiome influences these dynamics are still unclear (Fig. 2).
HPA, Hypothalamic-Pituitary-Adrenal; SCFA, short chain fatty acids; GABA, Gamma-Aminobutyric Acid.
Disturbances in the bidirectional communication between the gut and the brain can lead to various conditions, such as inflammatory bowel syndrome, and various chronic neurological conditions, including Parkinson’s disease, ASD, chronic pain and mood changes.40 The gut microbiome can affect the central nervous system (CNS) through multiple mechanisms, primarily by modulating the immune system through various cytokines and the release of metabolites, including neurotransmitters, by gut bacteria.13 Children with autism more frequently exhibit increased intestinal permeability. This may lead to greater interactions between the mucosal immune system and the lipopolysaccharides (LPS) of gut bacteria, inducing the production of cytokines such as IL-1β, IL-6, IL-8, and TNF-α. Elevated levels of immune modulators in plasma have been associated with neurodevelopmental disorders in children with autism.41,42 A study by Zurita et al. demonstrated a correlation between differences in the gut microbiome of children with autism and neurotypical children, with significantly elevated serum levels of TGF-β (thrombocyte-growth factor-β). No significant changes in the serum levels of IL-6 were noted.43 The bacterial genera most strongly associated with differences in cytokine profiles were Bacteroides, Prevotella, and Bifidobacterium.44 The central role of the immune system in mediating communication between the gut microbiome and the brain, as well as other peripheral systems, was further highlighted by Jacobson et al.39
During their metabolic processes, bacteria can release various substances that affect the CNS. For example, short chain fatty acids(SCFAs) such as acetic acid, propionic acid, and butyric acid are released. In children with autism, increased levels of acetate and propionate and decreased levels of butyrate are commonly observed. These changes may be related to neurodevelopment by directly affecting CNS physiology through increased epigenetic changes and/or mitochondrial dysfunctions.45,46 Elevated levels of another metabolic product, p-cresol, primarily released by Clostridium difficile and Bifidobacterium spp., are also associated with behavioral deterioration in children with autism.47 By inhibiting dopamine-β-hydroxylase, p-cresol can influence dopamine metabolism in the brain.48 A simulation study analyzing enzyme production involved in glutamate metabolism across different microbiomes revealed that these enzymes were less represented in the autistic microbiome compared to healthy microbiome. Glutamate is an important metabolite for neurological development. On one hand, it is a component of the peptide glutathione, which acts as an antioxidant to reduce oxidative stress in cells. On the other hand, glutamate is an excitatory neurotransmitter, and an imbalance in its activation and repression within the CNS may contribute to the development of ASD.49 The microbial population in the colon may also be related to other molecules that serve as neurotransmitters in the CNS. For instance, Lactobacillus spp. and Bifidobacterium spp. can produce Gamma-Aminobutyric Acid (GABA), a major inhibitory neurotransmitter which is found in increased concentrations in children with autism.50 Particularly important in the gut-brain axis is the neurotransmitter serotonin. Some species, such as Escherichia, Enterococcus, and Candida, can directly synthesize serotonin, while others, such as Clostridium spp., and Lactobacillus spp., may be involved in regulating its secretion.50,51 In children with autism, hyperserotoninemia is observed along with reduced levels of serotonin in the brain.52 Elevated 3-(3-hydroxyphenyl)-3-hydroxypropionic acid, 3-hydroxyphenylacetic acid, and 3-hydroxyhippuric acid in children with autism suggest disruptions in phenylalanine metabolism. These metabolites are associated with an abundance of Clostridia spp., which may exacerbate autistic behavior.53
One of the main pathways for gut-brain interactions is the vagus nerve (n. vagus). It is composed of approximately 20% efferent fibers, which regulate gastrointestinal, lung, and heart functions, and approximately 80% afferent fibers, which are responsible for transmitting visceral and somatic sensations.54 The vagus nerve has anti-inflammatory functions, interacting with the hypothalamic-pituitary-adrenal axis, the splenic sympathetic anti-inflammatory pathway, and the cholinergic anti-inflammatory pathway. These interactions lead to reduced inflammation in the gut and brain, enhanced immune regulation, stress regulation, neurodevelopment, and the maintenance of cognitive functions.55 Impaired anti-inflammatory functions of the vagus nerve may contribute to low-grade inflammation in the gut and brain, as reported in children with ASD.5 In turn, chronic gut inflammation may lead to vagus dysfunctions, such as disruption of the cholinergic anti-inflammatory pathway, altered vagal signaling, vagal hypersensitivity, reduced vagal tone, or neuroinflammation.56 Gut microbiota metabolites, such as SCFAs, play a key role in maintaining gut barrier function and possess immune-modulatory activity, which reduces inflammation. SCFAs, particularly butyrate, can enhance vagal tone, thereby reducing symptoms such as anxiety and depression.57 Gut microbiota can also directly affect brain functions, influencing mood and behavior via the vagus nerve, with microbiota metabolites such as SCFAs and neurotransmitters such as GABA, serotonin, and dopamine acting as mediators.58 Although the direct mechanisms are not yet fully understood, increasing evidence suggests a link between dysbiosis, autistic manifestations, and the vagus nerve as a critical component of these interactions.
Although the mechanisms underlying the gut microbiome-brain connection and its relevance to autism development are not yet fully understood, these studies highlight key pathways that warrant further exploration in future research.
Modulation of the microbiome by diet, probiotics and antibiotics in children with autism
Various factors can influence the formation of the gut microbiome, including diet, vitamin and mineral intake, and the use of prebiotics, probiotics and antibiotics. Following the discovery of the gut-brain connection, numerous studies have explored how modulating the microbiome could affect neurological functions, including in children with autism.
Prebiotics are described as “non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thereby improving host health”.59 In a study by Grimaldi and colleagues, galactooligosaccharides were shown to affect the production of SCFAs, lowering propionate and increasing butyrate, which may benefit children with autism. In addition, this prebiotic can affect brain dysfunction caused by the overproduction of GABA.49
Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”.60 Various strains and combinations of strains are being investigated for their potential beneficial effects on both GI symptoms and behavior in children with autism. In a study by Shaaban and colleagues, 30 autistic children who consumed probiotics containing Lactobacillus acidophilus, Lactobacillus rhamnosus and Bifidobacteria longum were followed for 3 months. The study revealed an improvement in GI symptoms and a reduction in the severity of autistic symptoms.61 Billeci et al. demonstrated an improvement in the brain function of children with autism following probiotic intake, as measured by beta- and gamma-wave activity through EEG.62 A reduction in frontopolar region power was found in the beta and gamma bands, which are often elevated in autistic children.63 In a case report examining a 12-year-old patient with autism, fewer GI complaints, and an improvement in autistic manifestations were also observed.64
The number of participants in clinical trials on this subject is limited, but various studies are being conducted in animal models to establish the exact mechanisms by which probiotics affect neurodevelopment. The difficulties in studying ASD patients have led to the development of animal models that mimic the clinical characteristics of these patients. Depending on the construction methods, genetic models (Table 2),47 environment-induced models and idiopathic models (Table 3) are distinguished.47,65 These models are based on the understanding that, in addition to genetic factors, environmental factors may also influence ASD development.66 Environmental factors include pesticides, drug consumption, heat, diet, and pollutants.67,68
Animal models of Аutistic Spectrum Disorders (ASD) – genetic models47
| Model | Function/Damage | Animals | ||
|---|---|---|---|---|
| GENETIC MODELS | Syndromic ASD genes | |||
| MECP2 | Starts or inhibits transcription; neuron maturation; regulated by development | Mouse, Rodent, Fish, Invertebrates | ||
| FMR1 | Involves in translation; affects neuronal proliferation and migration | Rodent, Fish, Invertebrates | ||
| SHANK3 | Promotes formation, maturation, and stability of dendritic spines | Mouse, Rodent, Fish, Invertebrates | ||
| TSC1/2 | Regulates mTORC1 pathway, neuronal differentiation, and Purkinje cell excitability | Rodent | ||
| UBE3A | Regulates neuronal homeostatic synaptic plasticity | Rodents | ||
| Non-syndromic ASD genes | ||||
| NLGNs | Regulates the formation of hippocampal neurons and post-glutamatergic synapse proteins | Rodents | ||
| NRXNs | Encodes neuronal transmembrane protein; interacts glial cells | Rodents | ||
| CHD8 | Controls epigenetic and transcript regulation; affects brain phenotype | Rodents, Fish | ||
| POGZ | Regulates neuronal development | Rodents | ||
| ANK2 | Affects axonal branching; regulates postnatal development of excitatory synapses | Rodents | ||
| MIR137 | Regulates neuronal gene expression and neurogenesis | Rodents | ||
Animal models of Аutistic Spectrum Disorders (ASD)–Environmental-induced and idiopathic models47
| Environmental factors | |||
|---|---|---|---|
| ENVIROMENTAL-INDUCED MODELS DRUG-INDUCED MODELS | VPA (Valproic acid) | Affects expression of BDFN mRNA in brain tissue | Rodents, Fish |
| PPA (Propionic acid) | Reactive astrocyte keratinization of brain tissue; microglia are activated; oxidative stress markers rise; glutathione declines | Rodents | |
| BPA (Bisphenol propan) | Changes in number of neurons and glia in the medial prefrontal lobe | Rodents | |
| Sevoflurane | Increases the number of apoptotic cells in brain; inhibits the axon development of hippocampal neurons | Rodents | |
| ENVIRONMENTAL-INDUCED MODELS | Maternal immune activation models (MIA) | Abnormal increase of offspring’s brain volume | Monkey, Rodents |
| Borna disease virus (BDV) models | Abnormal hippocampal and cerebellar development | Rodents | |
| Gut microbiota models | Regulation of neuroactive metabolites | Monkey, Rodents, Fish, Invertebrates | |
| Repeated cold temperature stress (RCS) models | Changes in neurotransmitter and corticosterone levels | Rodents | |
| Inbred line | |||
| IDIOPATHIC MODELS | BTBR T+Itpr3tf/J mouse model | Polymorphisms in the Kmo gene, which encodes urine 3-monooxygenase | |
| Inbred line BALB/cByJ mouse model | Reduced corpus callosum volume |
In a study by Abuaish et al. using rodent models, alterations in the ratios of Clostridium spp. led to a decrease in Clostridium perfringens and an increase in Clostridium cluster IV, resulting in improved social behavior in these models when treated with Bifidobacterium longum BB536.69 In another study on mouse models of ASD supplemented with Lactobacillus plantarum ST-III, an increase in beneficial Lachnospiraceae spp. and a decrease in Alistipes spp. were observed, which also led to improvements in social behavior.70 In a study by Tabouy et al., mouse models of ASD treated with Lactobacillus reuteri showed increased GABA-receptor gene expression and protein levels of GABRA1 in the hippocampus.71 Furthermore, Lactobacillus reuteri therapy has been found to increase oxytocin levels in the brain, potentially improving brain function and behavior by stimulating the vagus nerve.72 Ingesting Lactobacillus rhamnosus in BTBR mouse models of ASD led to increased levels of beneficial neuroactive compounds such as 5-aminovaleric acid and choline.73 Despite these promising results in animal models, further clinical studies are needed to identify the exact bacterial strains or combinations that can serve as adjunctive therapies for autism in children.
Another approach to modulating the gut microbiome is through antibiotics. Oral vancomycin is often discussed due to its poor absorption in the GI tract, resulting in minimal systemic effects, and its action specifically targeting gram-positive bacteria, including Clostridium spp.74 In a study by Sandler et al.,11 children with ASD who received oral vancomycin therapy were followed up. The results revealed an improvement in GI symptoms and behavior in eight of them.75 However, the effects of this therapy were short-lived, likely due to the survival and subsequent proliferation of Clostridium spp. spores.3 The findings of Sandler et al. are being further explored in animal models, where vancomycin has also been shown to impact autistic behaviors. The effects of vancomycin can be explained by its influence on the gut microbiome, which in turn affects the production of SCFAs and various bacterial metabolites, impacting the mitochondrial dysfunction observed in autism.76 On the other hand, the changes induced by vancomycin may also lead to alterations in the relationship between the intestinal microbiome and the immune system.77
Another therapeutic approach of interest in children with ASD is fecal microbiota transplantation (FMT). FMT involves the transplantation of fecal matter from healthy donors to recipients to restore the healthy balance of the gut microbiota. It is often used to treat conditions related to gut dysbiosis, such as Clostridium difficile infections. While more studies have been conducted in animal models, a few have included FMT in children with ASD. In a systematic review by Zhang et al., five studies were reviewed: two prospective open-label studies, two retrospective observational studies, and a case report. All of them reported improvements in behavioral symptoms and reductions in gastrointestinal symptoms.78 In a clinical trial by Kang et al., 18 children diagnosed with ASD received fecal matter transplantation two weeks after antibiotic treatment and intestinal clearance. Eight weeks after transplantation, improvements in both gastrointestinal and behavioral symptoms were observed.79 Similar findings were reported in another study involving 8 children with ASD, two years after FMT.80 The encouraging results from these studies warrant additional, larger double-blind clinical trials to establish precise guidelines for this treatment.
Limitations
This review highlights the influence of the gut microbiome on the gut-brain axis and its role in ASD, while also presenting some potential therapeutic approaches. However, several limitations must be acknowledged. Тhe potential therapeutic approaches have been studied in different studies with small sample sizes and short-term observations, which may not provide a comprehensive understanding of long-term effects. Larger double-blind placebo-controlled trials are necessary to confirm the effects of these treatments and establish exact guidelines. Additionally, studies on gut microbiome abnormalities have been conducted on a relatively small number of participants, which may affect the reliability of their results. When tracking changes in the microbiome, it is important to consider the specific characteristics of the microbiome in different populations, which are influenced by geographical location, environmental factors, local diet, and traditions. To more accurately identify differences in the microbial profile of children with autism, studies involving larger participant numbers and focused on specific populations are needed. Notably, the mechanisms of interaction between the gut microbiome and the gut-brain axis in ASD are still not fully understood. Our review reflects the main findings to date; however, further research is necessary to clarify these relationships fully and comprehensively.
Conclusions
The role of the gut microbiome in health and disease is garnering increasing interest, particularly in its impact on neurological disorders such as ASD. ASD is socially significant because of its chronic nature and the limited number of effective therapeutic approaches available. Research suggests a connection between the gastrointestinal tract and neurological disorders, with evidence showing more frequent gastrointestinal symptoms in children with autism. Advances in microbiome research have revealed differences in microbial profiles between children with autism and neurotypical children. Factors such as birth method, diet, and the use of antibiotics by either the child or the mother during childbirth or pregnancy can influence the composition of the microbiome. Managing these factors may offer preventative benefits and help reduce the incidence or severity of such conditions in children. The metabolic byproducts of gut bacteria can affect the central nervous system directly or by influencing other metabolic processes, leading to changes at various molecular levels. Understanding the precise mechanisms by which the microbiome influences the gut-brain axis through its metabolites is crucial. Positive outcomes from modulating the gut microbiome via the use of probiotics, antibiotics, or fecal microbiota transplantation present promising new therapeutic options for addressing neurological and gastrointestinal disorders. However, this area of research is still developing, and more studies with larger sample sizes are needed to establish precise guidelines.
Declarations
Acknowledgement
The authors have no individuals or organizations to acknowledge in relation to this article.
Funding
The authors received no specific funding for this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Authors’ contributions
Review’s concept and design (VP, AK), acquisition of data (VP, IP, DD, EA), analysis and interpretation of data (VP, IP, DD, EA), drafting the manuscript (VP, IP, DD, EA), critical revision of the manuscript (AK), technical support (AK), and study supervision (AK) were performed. All the authors have approved the final manuscript.

 Author information
Author information