Introduction
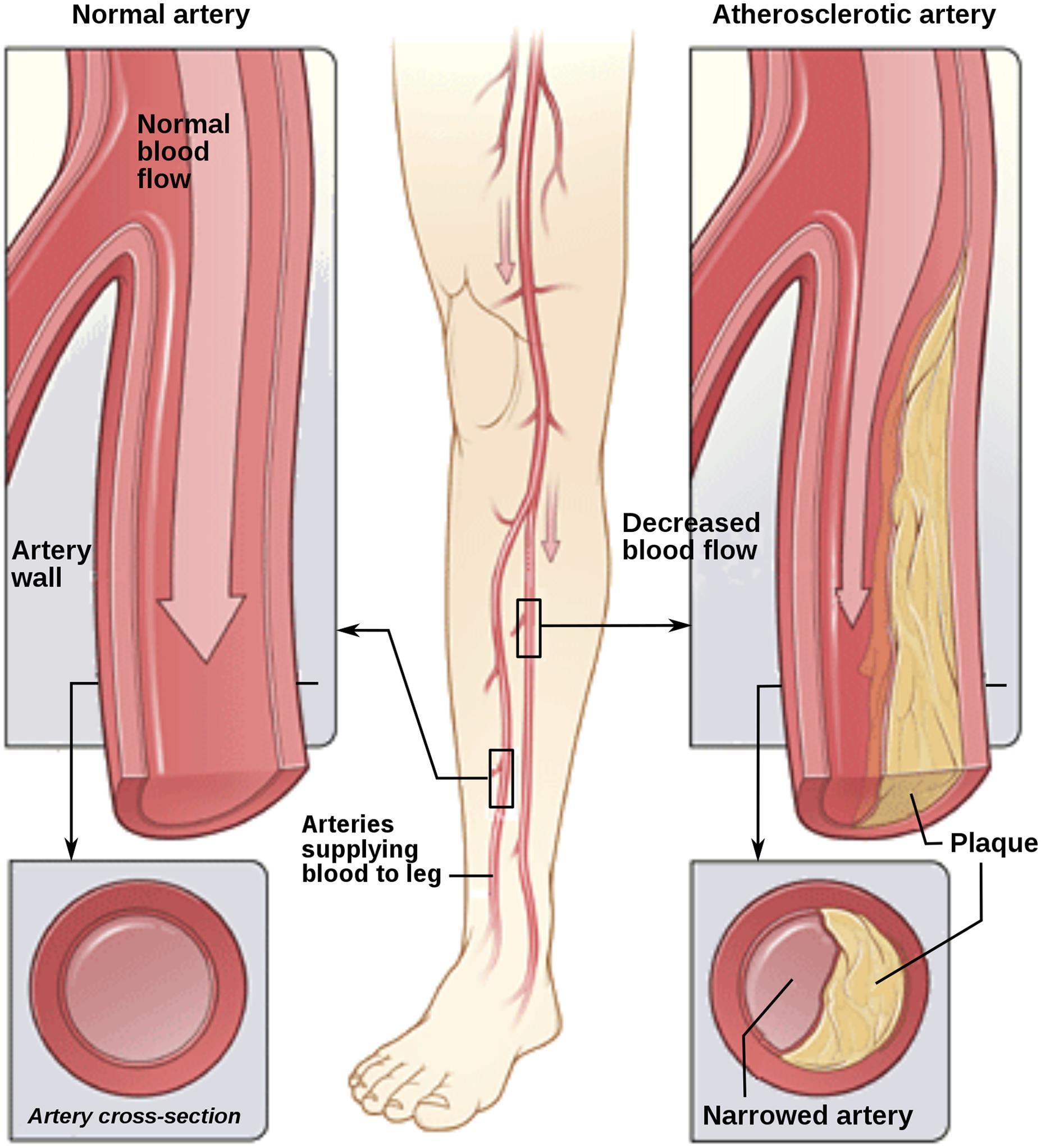
Peripheral arterial disease (PAD), a global public health concern, is increasingly prevalent and poses serious risks. It is characterized by reduced blood flow to the extremities, leading to complications beyond vascular insufficiency (Fig. 1). The disease is a major contributor to morbidity and mortality, with recent statistics indicating a rising trend in its prevalence, particularly among the aging population. The global prevalence of PAD has shown significant increases over the years, as described in the Global Burden of Disease Study 2019 database, a systematic analysis of 369 diseases and injuries in 204 countries and territories published in The Lancet in 2020.1 As of 2019, there were approximately 113.4 million prevalent cases of PAD worldwide, a substantial increase from the 65.7 million cases estimated in 1990.1 The prevalence per 100,000 persons increased by 13% during this period, although the age-standardized prevalence rate showed a decline of 22%.1 This decline in age-standardized rates suggests some progress in managing the condition, possibly due to advancements in healthcare and increased awareness.1 The mortality associated with PAD also presents a concerning picture. There was a 1.47-fold increase in PAD-related deaths globally, rising from 30,000 deaths in 1990 to about 74,100 in 2019.2–4 Current treatments, which are largely centered around lifestyle changes and pharmacotherapy, often fall short in addressing the complex nature of PAD. This underscores the necessity for innovative therapeutic approaches. The emerging field of arteriogenesis, which focuses on the growth and remodeling of collateral vessels in response to fluid shear stress (FSS), offers a promising avenue. Recent studies have illuminated the role of MicroRNAs (miRNAs) as crucial regulators in this process, making them a target of significant interest in PAD management.4–8
This illustration shows how PAD can affect arteries in the legs. The left image depicts a normal artery with unobstructed blood flow. The inset shows a cross-section of the normal artery. The right image shows an artery with plaque buildup, partially blocking blood flow. The inset shows a cross-section of the narrowed artery. (Copyright: Jmarchn. This file is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license).
The multifaceted nature of PAD demands a holistic understanding of the molecular, cellular, and physiological dynamics involved. The convergence of fluid shear stress, miRNA biology, and arteriogenesis unfolds as a promising frontier, beckoning researchers and clinicians alike to unlock the full therapeutic potential within this intricate tapestry of vascular biology.
Arteriogenesis and FSS
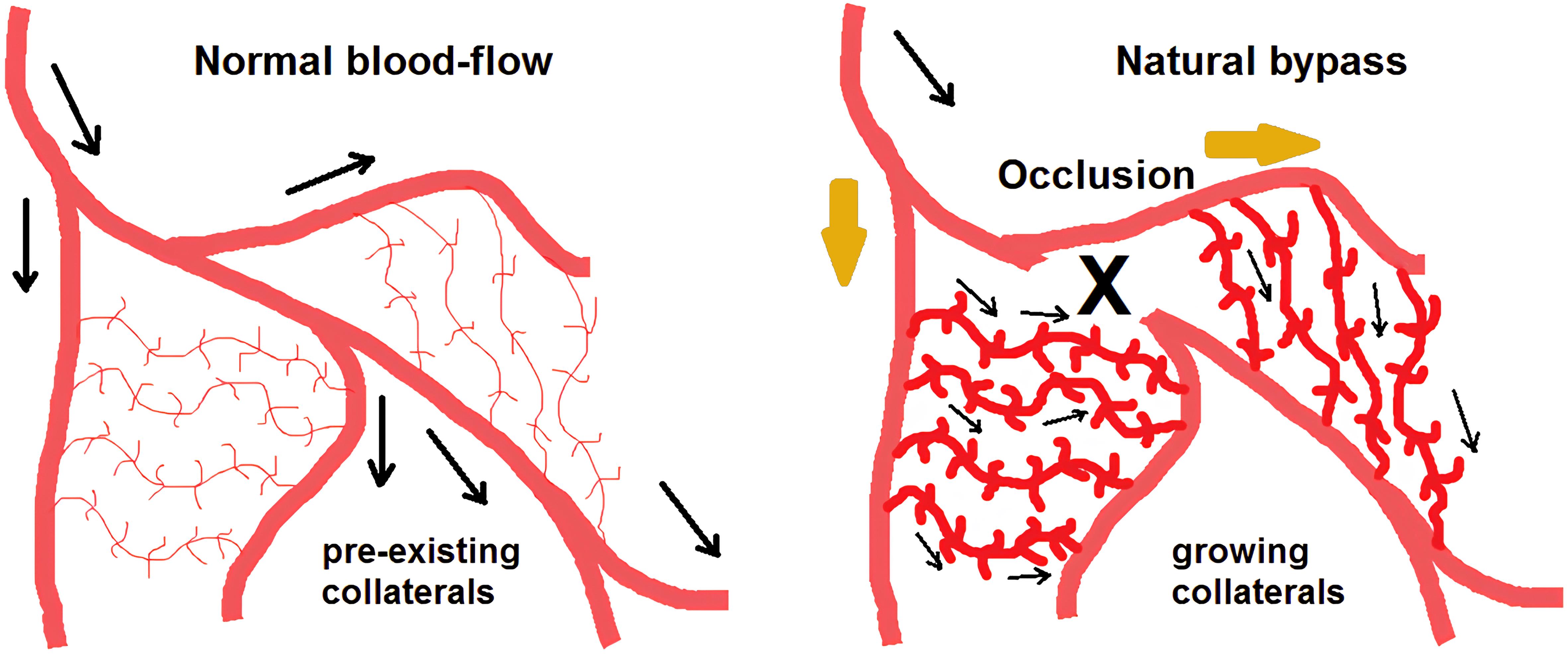
Arteriogenesis, a natural physiological response to hemodynamic changes caused by FSS, is key in the development of collateral vessels (Fig. 2).9–12 This process is critical for maintaining blood flow despite arterial blockages. A deeper understanding of the molecular pathways, particularly the role of miRNAs in response to FSS, can lead to groundbreaking therapeutic interventions in PAD.
This illustration depicts the active remodeling of non-functional vascular anastomoses into functional collateral arteries. These arteries are capable of bypassing the site of obstruction and preserving the tissue that is jeopardized by ischemia. (Adapted from: Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ Res. 2004 Sep 3;95(5):449-458).
miRNAs in atherosclerosis and arteriogenesis
Several recent studies have demonstrated that miRNAs play a critical role in the development of atherosclerosis, encompassing conditions such as coronary artery disease, PAD, and carotid artery disease. There is a direct link between miRNAs and the severity of a patient’s atherosclerosis, as well as their functional prognosis, indicating that miRNAs could serve as valuable biomarkers, especially in patients with coronary artery disease for diagnosing and evaluating the prognosis of cardiovascular events (Table 1).13 Current evidence suggests that certain miRNAs may have diagnostic or prognostic value in identifying vulnerable plaque characteristics or predicting future ischemic events (Table 2).14 During different stages of atherosclerosis, various miRNAs can be considered potential targets for therapy, as they regulate specific phases of atherosclerotic development. In the early stages of atherosclerosis, one promising target is miR142-3p, which could play a role in both preventing and treating this condition.13 Additionally, miR92a presents an intriguing target due to its association with plaque progression and instability.13 It is important to exercise caution with individual miRNAs, as their roles can vary depending on their origin or the stage of atherosclerosis. For instance, miR21, miR155, and miR33a/b regulate cholesterol homeostasis and oncogene expression and thus warrant careful consideration.13 The presence of PAD lesions has long been associated with tobacco smoking. In a study by Pereira-da-Silva et al., active smokers, as opposed to former smokers, exhibited a decrease in miR27b levels, which was linked to the presence and severity of PAD. These findings imply that miR27b plays a role in the proatherogenic impacts of cigarette smoking and that quitting smoking may be associated with a reduction in miR27b dysregulation.15
Overview of the most clinically useful miRNAs in CAD, detailing their functions, source locations, expression levels, and target molecules13
| Proposed role | miRNA(s) | Up- or down-regulated | Diagnostic (D) vs. Therapeutic (T) potential |
|---|---|---|---|
| Expressed in many cells | |||
| Highly expressed in VSMCs, ECs, cardiac fibroblasts, cardiomyocytes, and platelet apoptosis and eNOS activity | miR21-5p | Up | D, up-regulated in CAD patients compared to controls (AUC: 0.767, p < 0.001) |
| Platelets | |||
| Humans: collagen-induced platelet aggregation; Mice: expression of the P2Y12 receptor | miR126-3p | Up | D, monitors P2Y12 inhibition |
| Responsive to antiplatelet therapy | miR126-3p | Up | T, an antagomir against miR126-3p reduces platelet aggregation |
| Marker of platelet activation, which targets the COX1 receptor through the regulation of TXS | miR34b-3p | Up | D, miR34b-3p may facilitate the antiplatelet efficiency of aspirin by inhibiting TXS |
| Marker of response to clopidogrel, that targets the P2Y12 receptor | miR223-3p | Down | D, high on-clopidogrel platelet reactivity |
| miRs released by platelets, which are responsive to antiplatelet therapy | miR126 miR150 miR191 miR223 | Up Up Up Up | D, antiplatelet therapy significantly reduces their levels |
| VSMCs | |||
| High expression is needed to maintain a contractile phenotype of VSMCs | miR22 | Down | T, a stent with an miR22 coating showed significant capability to inhibit in-stent restenosis (an animal study) |
| Mitigates atherosclerosis, VSMCs contractility, increases fibrous cap area, and reduces the necrotic core area | miR145 | Down Down | T, delivery of miR145 may limit atherosclerotic plaque growth, and restore contractile levels in VSMCs |
| Down-regulation of miR-145 plays a critical role in the pathogenesis of atherosclerotic plaques, and neointimal hyperplasia | miR145 | Down | D, reduced plasma miR145 levels correlate with an increase in CAD severity (SYNTAX score) |
| Induces VSMC senescence, promotes the expression of age-associated pro-inflammatory secretory factors, and increases the binding capacity of ox-LDL to macrophages | miR34a | Up | D, increased expression in CAD, compared to healthy controls (AUC: 0.899, p < 0.001), associated with Gensini score (p < 0.001) |
| Arterial endothelial cells | |||
| Plays a crucial anti-atherogenic role by regulating the function of ECs and enhancing endothelial repair | miR126-3p | Down | D, reduced expression is associated with more severe and complex CAD |
| Decreases size of atherosclerotic lesions, alleviates ox-LDL-induced EC injury | miR126-3p | Down Up | D, decreased expression in CAD patients, compared to healthy controls but up-regulated in ACS |
| Induces EC apoptosis, development of atherosclerosis | miR142-3p | Up | T, down-regulation of miR142-3p suppresses ECs apoptosis |
| Induces apoptosis and oxidative stress, and is pro-atherosclerotic | miR92a-3p miR486 | Up Up | D, discriminate between stable and vulnerable CAD |
| Lipid metabolism | miR122 | Up | D, increased in CAD patients, and with CAD severity (Gensini score) |
| Recovery of ischemic tissue | miR17 | Down | D, reduced expression is associated with more severe and complex CAD |
| Rate of apoptosis in ECs | miR17-5p | Up | T, inhibition of miR17 suppresses apoptosis, hence, decreases infarct size area, and improves microcirculation of heart tissue, decreasing heart failure symptoms |
| Macrophages | |||
| Regulator of cholesterol and fatty acid homeostasis, reverse cholesterol transport, increases HDL-cholesterol level | miR33a miR33b | Up Up | T, inhibition of miR33a facilitates atherosclerotic regression |
| Inhibits oxidized LDL-induced lipid accumulation and inflammatory response | miR146a | Up | D, patients with stable CAD had a 3.62-fold higher expression level, compared to controls (AUC:0.767) |
| Reduced in diabetics, has a role in lipid metabolism | miR155 | Down | D, patients with stable CAD had 1.89-fold lower expression level, compared to controls (AUC:0.767) |
| Cardiomyocytes | |||
| Indicates myocardial damage | miR223-5p | Up | D, increased expression, compared to the healthy control group, with an AUC of 0.933 for predicting CAD severity |
| Suppresses EC proliferation rate, viability, and migration activity involved in heart development, and indicates myocardial damage | miR133a | Up | D, increased expression in CAD, compared to healthy controls, but with a low AUC of 0.597 correlates with the Gensini score of CAD severity (r = 0.303, p = 0.007) |
| Indicates myocardial damage; Indicates myocardial damage; Fibrous cap increase; Indicates myocardial damage; Fibrous cap thinning; Plaque neovascularization; Indicates myocardial damage | miR133a miR182 miR145 miR205 miR208a miR21 miR126 miR223 | Down Up Up Up Down Up Up Up | CAD vs. controls: AUC: 0.863, p < 0.001 AUC: 0.959, p < 0.001 AUC: 0.836, p < 0.001 AUC: 0.959, p < 0.001 AUC: 0.616, p = 0.015 AUC: 0.767, p < 0.001 AUC: 0.767, p < 0.001 AUC: 0.616, p = 0.015 |
| Induces angiogenesis and myocardial damage; Indicate myocardial damage | miR1 miR133a | Up Up | D, increased expression, compared to a healthy control group |
| Expressed in myocardial cells | miR23a | Up | D, up-regulated, positive correlation with CAD severity |
| Cardiac myofibroblast differentiation, smooth muscle cell modulator, increases fibrous cap area, reduces necrotic core | miR145 | Down | D, reduced in patients with cardiac ischemia |
| Indicates myocardial damage, cardiac hypertrophy; Protects against H2O2-induced apoptosis | miR208b miR499 | Up Up | D, independent predictors of a high SYNTAX score miR208b: AUC: 0.775, p < 0.001 miR499: AUC: 0.713, p < 0.001 |
| Increases foam cell formation | miR23a | Up | D, correlates with CAD severity (Gensini score) |
miRNAs associated with carotid plaque instability and vulnerability. Comparison of the expression of miRNAs in symptomatic and asymptomatic atheromatous plaques in patients with carotid artery disease14
| Studied groups | miRNA(s) | Up- or down-regulated | Rationale behind the selection of this miRNA(s) | Statistical significance/AUC, or HR (95% CI), p-value |
|---|---|---|---|---|
| 15 sAPs and 15 aAPs | miR100 miR125a miR127 miR133a miR145 miR221 | Up Up Up Up Up Up | miRNAs involved in plaque growth and instability | p < 0.001 p < 0.001 p < 0.001 p < 0.001 p < 0.001 p < 0.001 |
| 22 sAPs and 31 aAPs | miR145 miR133a | Up | large-scale analysis | p = 0.027 p = 0.044 |
| 45 sAPs and 31 aAPs | miR221 miR222 | Down Down | miRs promoting VSMC proliferation and intimal thickening | p < 0.001 p < 0.001 |
| 12 sAPs and 10 aAPs | miR200c | Up | induction of endothelial dysfunction, ROS production | p < 0.001 |
| 20 sAPs qualified as stable vs. unstable in histology examination | miR330-5p | Up | regulating effect of miR330-5p on Talin1 mediator | p < 0.05 |
Recent research has established miRNAs as central players in FSS-induced arteriogenesis. These small non-coding RNAs modulate gene expression and have been implicated in various aspects of vascular biology. In the present article, we have synthesized findings from multiple studies to elucidate the roles of miRNAs,4–8 particularly in the context of FSS-induced arteriogenesis, and how they might be harnessed for PAD therapy (Table 3). While much attention has been given to miR143-3p, other miRNAs also play significant roles in arteriogenesis. For instance, miR210 and miR126 have been implicated in angiogenic processes, suggesting their potential roles in PAD therapy. A comprehensive analysis of these and other relevant miRNAs can provide a more rounded understanding of the miRNA landscape in arteriogenesis.
Summary of recent experimental studies analyzing the role of different miRNAs in atherosclerosis and FSS-driven arteriogenesis, and their potential clinical implications in PAD therapy4–8
| Study–first author (year of publication) | Study type | miRNA(s) studied | Role in arteriogenesis and atherosclerosis | Potential role in PAD therapy |
|---|---|---|---|---|
| Sieland J. et al. (2023)4 | Two-armed, randomized- balanced cross-over study | miR142-5p, miR424-5p | miR142-5p is associated with VEGF and mTOR signaling in smooth muscle cells and induces apoptosis in human macrophages by targeting TGF-β2. miR424-5p plays an important role in regulating cell-intrinsic angiogenic activities of endothelial cells. | miR142-5p could play an important role in the progression of atherosclerosis. miR424-5p could improve vascular endothelial function and peripheral blood circulation. |
| Sieland J. et al. (2021)5 | Three-arm, randomized- balanced cross-over study | miR142b- 5p, miR197-3p, miR342-3p, miR424-5p | miR142b-5p (see above). miR197-3p regulates endothelial cell proliferation via targeting VEGF. miR342-3p represses inflammation in atherosclerosis. miR424-5p regulates HIF-α isoforms and promotes angiogenesis (also see above). | miR142b-5p could be a factor in hypertrophy and improvement of the cardiovascular system and endurance performance (also see above). miR197-3p has shown a predictive value for myocardial infarction. miR342-3p alters the immune system response and minimizes inflammation during aerobic exercise. miR424-5p improves vascular endothelial function and peripheral blood circulation during exercise. |
| Troidl K. et al. (2020)6 | Experimental interventional study | miR143-3p | miR143-3p targets the extracellular matrix protein collagen type V-α2 during collateral vessel remodeling. | miR143-3p could directly impact collateral artery growth leading to restoration of blood flow after femoral artery occlusion. |
| Vogel J. et al. (2019)7 | Three-arm, randomized- balanced cross-over study | miR143-3p | miR143-3p is an essential factor for proper collateral formation following femoral artery ligation. It plays a pivotal role in smooth muscle cell differentiation and vascular disease. | Training intensity maximizes the beneficial alternative to invasive therapies for PAD patients. |
| Guan Y. et al. (2017)8 | Experimental interventional study | miR352 | miR352 is expressed in endothelial cells and inhibits arteriogenesis in vivo and angiogenesis in vitro via regulating its target gene IGF2R/CI-M6P. | Downregulation of miR352 expression may promote collateral artery growth. |
Mechanotransduction events in arteriogenesis
Understanding mechanotransduction events in arteriogenesis is crucial for identifying potential therapeutic targets. In a mouse model of femoral artery ligation, increased FSS prompted endothelial cells to release extracellular RNA (eRNA). Notably, administering RNase inhibitors improved perfusion recovery, highlighting the regulatory role of eRNA in leukocyte recruitment and collateral artery growth. The engagement of vascular endothelial growth factor receptor 2 (VEGFR2) by eRNA emerged as a key mechanistic link, emphasizing the intricate signaling cascade initiated by FSS.16
eRNA plays a pivotal role in arteriogenesis by regulating leukocyte recruitment and collateral artery growth.5 In the mouse model of femoral artery ligation, endothelial cells released eRNA in response to increased FSS. Administration of RNase inhibitors, which block plasma RNases and prevent the degradation of eRNA, significantly improved perfusion recovery. In contrast, treatment with bovine pancreatic RNase A or human recombinant RNase1 interfered with leukocyte recruitment and collateral artery growth. These findings suggest that eRNA is a critical mediator of mechanotransduction in arteriogenesis.17
Further studies using a murine cremaster model of inflammation confirmed that eRNA regulates leukocyte recruitment by engaging VEGFR2. Intravital microscopic studies revealed the intricate role of eRNA in initiating the multistep inflammatory process responsible for arteriogenesis. Moreover, the release of von Willebrand factor as a result of shear stress was found to be dependent on VEGFR2. Blocking VEGFR2, RNase application, or von Willebrand factor deficiency interfered with platelet–neutrophil aggregate formation, highlighting the essential role of eRNA in the inflammatory process during arteriogenesis.16,17
Exercise and shear-stress-sensitive channels
The importance of shear-stress-sensitive calcium channels, including Trpv4, for cerebral arteriogenesis was investigated in a prospective controlled study that analyzed the expression profiles of shear-stress-sensitive channels, comparing the stimulation of collateral growth by target-specific drugs to that achieved by maximum increased FSS.18 Trpv4 showed significantly increased mRNA abundance and protein expression after FSS stimulation. Pharmacological activation of Trpv4 enhanced cerebral arteriogenesis to almost the same extent as maximum FSS stimulation, pinpointing Trpv4 as a potential candidate for new therapeutic concepts.18
The comparison between exercise-induced arteriogenesis and arteriovenous shunt models provided insights into the importance of shear-stress-sensitive channels. While collateral formation was greater in the shunt group, exercise-induced up-regulation of the transient receptor potential cation channel, subfamily V, member 4 (Trpv4) demonstrated a significant impact.19 Rats were subjected to femoral artery occlusion, followed by either exercise or arteriovenous shunt. Collateral formation was greater in the shunt group than in the exercise group, indicating the importance of chronic increases in fluid shear stress for sufficient arteriogenesis. Interestingly, exercise-induced Trpv4 up-regulation returned to control values six hours post-exercise, emphasizing the need for consistent and frequent exercise to maintain the stimulus for arteriogenesis.19
Exercise-induced miRNA dynamics in PAD
Investigating the acute effects of exercise on miRNA parameters related to vascular collateral formation provides valuable clinical insights. In a randomized crossover study involving patients with PAD, moderate-intensity walking demonstrated a favorable miRNA profile, including up-regulation of miR142-5p and miR424-5p.4 These miRNAs, associated with endurance training, showcased the potential of moderate exercise in promoting vascular health. The differential response observed in vigorous-intensity walking emphasized the importance of optimizing exercise regimens for personalized therapeutic outcomes.4
Exercise has long been established as a preferred therapy for PAD in its early stages. A recently published study focused on the acute effects of a single bout of aerobic and anaerobic walking on miRNA parameters related to vascular collateral formation in patients with PAD.5 Ten participants underwent two intervention arms: one involving vigorous-intensity walking on a treadmill to volitional exhaustion, and the other involving moderate-intensity walking at an individually selected speed for 20 m. The results indicated that vigorous-intensity walking led to a higher heart rate and increased lactate concentration, while moderate-intensity walking resulted in the up-regulation of miR142-5p and miR424-5p.5
These findings suggested that moderate-intensity walking may be more feasible than vigorous exercise in inducing changes in blood flow and endurance training-related miRNAs in patients with PAD.5 The data implied that the effect mechanisms might follow an optimal rather than a maximal dose-response relation. Steady-state walking without reaching exhaustion appeared to be better suited as a stimulus for promoting vascular health in PAD.5
Role of miR143-3p and other miRNAs in arteriogenesis
Specific miRNAs play pivotal roles in FSS-induced arteriogenesis. miR143-3p, identified as a key regulator, exhibited increased expression in collateral tissue following femoral artery ligation. Functional studies using in vivo mouse ligation models emphasized the critical role of miR143-3p in blood flow recovery and collateral artery growth. Mechanistically, FSS-induced expression of miR143-3p was linked to the release of transforming growth factor-β, contributing to vascular remodeling processes.6
Investigation into the effects of blood flow restriction on exercise-induced arteriogenesis revealed the importance of miRNAs, particularly miR143-3p. In a three-arm, randomized crossover study involving healthy volunteers, different resistance training interventions were compared.7 The results showed that the high-intensity intervention resulted in a higher lactate concentration and down-regulation of miR143-3p, while the low-intensity blood flow restriction protocol led to up-regulation of miR143-3p. The strong effects of both interventions on lactate- and arteriogenesis-associated miR143-3p in young and healthy athletes highlight the significant role of this miRNA in metabolic processes during exercise-induced arteriogenesis.
Moreover, the study explored the effects of different approaches to endurance exercise on circulating miRNAs.7 The three-arm, randomized crossover study involved treadmill-based acute endurance exercise at different intensities. The results indicated that all training interventions increased lactate concentration and heart rate. High-intensity exercise resulted in a higher lactate concentration than both lower-intensity protocols. The levels of specific miRNAs were altered in response to different exercise intensities, emphasizing the importance of both exercise intensity and type in modulating miRNA expression patterns.
Another study investigated the role of miR352 in regulating collateral vessel growth under conditions of elevated FSS.8 The research found that miR352 expression is downregulated in response to increased FSS in rat hind limbs. This downregulation promotes collateral vessel growth, as evidenced by the fact that inhibiting miR352 through antagomir-352 increased the number and proliferation of collateral vessels in a rat hind limb ligation model.8 In cell culture studies, miR352 inhibition led to increased endothelial proliferation, migration, and tube formation. The study also identified insulin-like growth factor II receptor (IGF2R) as a potential target influenced by miR352, suggesting a complex pathway for arterial growth regulation.8
Often referred to as a “master regulator” of hypoxia, miR210 is known to modulate various genes involved in cell survival, angiogenesis, and mitochondrial metabolism. It is upregulated in response to hypoxic conditions and contributes to the formation of new blood vessels, an essential process in restoring blood flow in ischemic tissues.20
Another miRNA, miR126, is predominantly expressed in endothelial cells and is crucial for vascular integrity and angiogenesis. miR126 influences several aspects of endothelial cell behavior, including cell migration and growth, which are critical for the development of new blood vessels.21
Future research could focus on investigating the synergistic or antagonistic interactions among these key miRNAs in the context of arteriogenesis. Specifically, exploring how these miRNAs collectively regulate various stages of vascular remodeling, collateral vessel growth, and blood flow recovery under different physiological stimuli could provide valuable insights into the intricate network of miRNA-mediated arterial growth. Additionally, understanding the crosstalk between different miRNA-regulated pathways and identifying potential target genes common to multiple miRNAs may offer a more comprehensive understanding of the regulatory mechanisms underlying arteriogenesis.
Translating miRNA research to clinical applications
Translating miRNA research into practical treatments presents several challenges. Delivery methods, such as nanoparticle-based systems and pharmacological preparations, need to be optimized for effective and safe miRNA modulation in patients. Limitations and challenges primarily include issues related to the delivery of miRNA-based therapies, ensuring their stability and targeted action within the human body, and overcoming potential side effects. Various delivery methods are being explored, including viral vectors, lipid-based nanoparticles, and exosomes. These methods need to be optimized to ensure efficient and targeted delivery with minimal side effects. Additionally, miRNA therapies must be designed to regulate gene expression effectively without triggering unintended effects on non-target genes. Clinical trial designs focusing on miRNA-based therapies are still in their infancy, requiring robust frameworks to assess efficacy and safety. Key endpoints in PAD trials could include pain-free walking distance, limb salvage rates, or quality of life measures. Statistical analysis of these endpoints helps in understanding the effectiveness of miRNA-based therapies. Data from randomized controlled trials (RCTs) involving miRNA therapies can provide robust evidence of their efficacy and safety. Following patients over time can provide data on long-term effects and potential complications of miRNA therapies. Analyzing data based on patient subgroups (e.g., based on disease severity, age, and comorbidities) can reveal which groups benefit most from miRNA therapies. Aggregating data from multiple studies can give a broader understanding of the effectiveness and safety of miRNAs in PAD. These complexities necessitate careful consideration in the development of therapeutic strategies and robust clinical trial designs to ensure safety and efficacy.22
Therapeutic potential of miRNAs
Developing practical applications of miRNA therapies in PAD involves not only understanding their mechanisms but also creating actionable strategies for patient treatment. This includes identifying specific miRNAs as therapeutic targets, optimizing delivery methods, and designing clinical trials to evaluate their efficacy in PAD management. Along with the scientific and clinical aspects, there are regulatory and ethical issues that must be addressed, including obtaining regulatory approvals, ensuring ethical standards in trials, and considering the long-term implications of gene regulation therapies. Harnessing miRNAs for practical patient benefit in therapeutic applications could ideally involve the following steps: (a) identifying specific miRNAs associated with disease pathology and validating their roles as potential therapeutic targets, (b) developing miRNA mimics or inhibitors that can modulate the activity of target miRNAs. This involves designing molecules that can either inhibit overactive miRNAs or replace underactive ones, (c) developing effective and safe delivery systems for miRNA therapeutics, (d) utilizing miRNAs in precision medicine by tailoring treatments based on individual miRNA profiles, which could involve using miRNA profiling to predict disease progression or response to therapy, (e) conducting rigorous clinical trials to assess the safety, efficacy, and optimal dosing of miRNA-based therapies, and (f) exploration of the potential of miRNA therapies in combination with existing treatments to enhance therapeutic efficacy or reduce side effects.23
The exploration of miRNA therapies in the realm of PAD opens new horizons, holding the potential to revolutionize vascular health and inspire optimism for enhanced clinical outcomes and improved patient well-being. As research progresses, the confluence of mechanotransduction, miRNAs, and therapeutic modalities is likely to reshape the landscape of PAD management, ushering in an era characterized by precision medicine in cardiovascular health.
Declarations
Acknowledgement
None.
Funding
There was no funding received.
Conflict of interest
The author declares no conflict of interest.
Authors’ contributions
The sole author contributed everything to the manuscript.
 Author information
Author information