Introduction
Breast cancer is a malignant tumor with the highest morbidity rate among women worldwide.1,2 Triple-negative breast cancer (TNBC), a subtype of breast cancer that is negative for estrogen, progesterone, and the human epidermal growth factor-2 (HER-2), accounts for 15–24% of all breast cancer cases.3–6 Predominantly affecting young women, TNBC is characterized by large tumor sizes, high tumor grades, a greater likelihood of lymph node metastasis, high invasiveness, strong heterogeneity, and high propensity for recurrent metastasis, making it the subtype with the worst prognosis. The 5-year disease-free survival rate for early-stage TNBC is approximately 70%, but once recurrent metastasis occurs, the median survival time is only 1–2 years.7,8 In recent years, notable progress has been witnessed in the pharmaceutical treatment of TNBC, notably through the use of immune checkpoint inhibitors (ICIs). The primary ICIs currently available are monoclonal antibodies targeting the programmed cell death receptor-1 (PD-1), the programmed cell death ligand-1 (PD-L1), and the cytotoxic T-lymphocyte-associated antigen 4 (CTLA4). Since PD-1 and PD-L1 inhibitors are the most common drugs used in breast cancer immunotherapy, this paper will primarily review the advancements in the treatment of TNBC with PD-1/PD-L1 monoclonal antibodies.
Predictive markers for the efficacy of ICIs
PD-L1
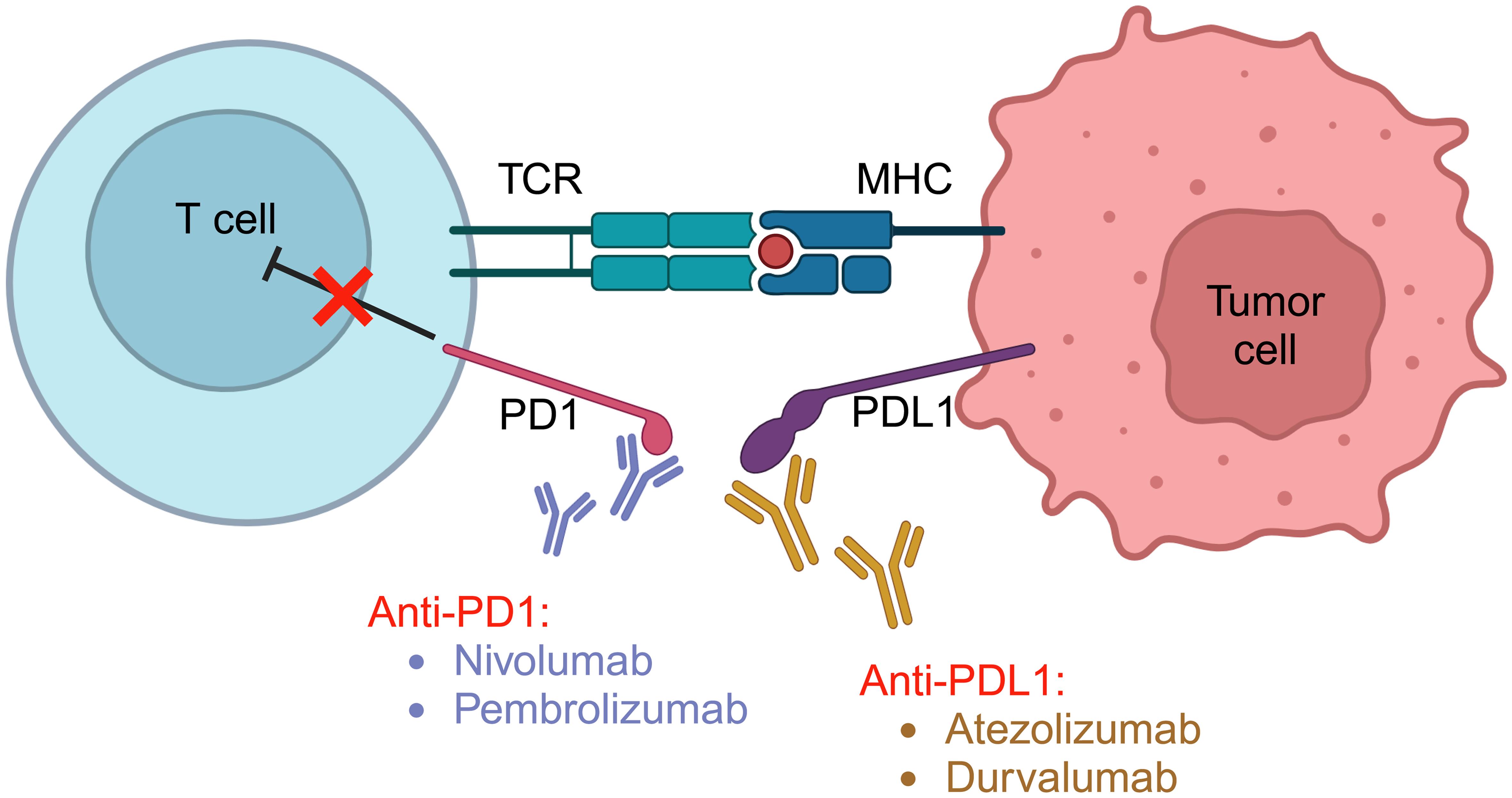
PD-L1 is a critical protein that regulates immune responses, particularly in pregnancy, allergy, autoimmunity, infection, and various physiological settings.9–11 This transmembrane protein, encoded by the CD274 gene in humans, is widely expressed in various cell types, including T cells, B cells, macrophages, dendritic cells, non-hematopoietic cells, and various tumor cells. PD-L1 interacts with its receptor, PD-1, found on activated T cells, leading to the inhibition of T cell responses (Fig. 1). The PD-1/PD-L1 interaction serves as a crucial immune checkpoint, preventing over-activation and maintaining immune response balance. In conditions like cancer, misregulation of PD-L1 allows diseased cells to evade the immune system.9 PD-1 is an immune checkpoint on the surface of T cells, and T cells can activate the immune response through multiple mechanisms after inhibiting PD-1.12 Firstly, PD-1 inhibition promotes the proliferation of T cell clones with lower affinity for neoantigens in tumors (i.e. suboptimal clones), increasing the number of T cells. These new T cells may replace T-cells that have infiltrated into the tumor site earlier, and become a potent force in the fight against tumors. Secondly, cross-reactivity occurs between the T cell receptor (TCR) and different antigens while inhibiting PD-1 activation in a large number of suboptimal clones, which may continue to cross-react with a variety of different antigens in the tumor. Additionally, suboptimal cloned T cells generally express fewer immune checkpoint molecules, are more likely to be persistently activated after PD-1 inhibition and are less likely to be completely depleted, which is more conducive to long-term efficacy.
In the context of cancer treatment, PD-L1 has become a significant biomarker. The presence of PD-L1 on tumors is used to predict responders to PD-1/PD-L1 checkpoint inhibitors, a class of drugs increasingly used in cancer immunotherapy. However, determining the correlation between PD-L1 expression and patient outcomes is not always straightforward, remaining a subject of ongoing investigation in cancer research. The Keynote-355 and Impassion130 studies showed that patients with PD-L1-positive metastatic TNBC (mTNBC) benefited from combined ICI treatment.13 However, findings from Keynote-522 and Impassion 031 studies conducted in patients with early-stage TNBC, revealed that the clinical benefit of combined ICIs was independent of PD-L1 expression.14–16 This incongruity has led to a current debate regarding the utility of PD-L1 as a predictive marker for the efficacy of ICI treatment. The selection of benefitting populations varies in detection methods, scoring systems, and positive thresholds. For instance, in the Impassion 130 study, 40.9% of patients were PD-L1 immune cell-positive, while the proportion of PD-L1 tumor cell-positive patients accounted for only 8.7% of the population.17
Tumor mutational burden (TMB)
TMB is a vital genomic marker that quantifies the total number of mutations within a tumor’s genome.18 It indicates the tumor’s mutation load and can predict the cancer’s response to immunotherapy, especially ICIs. A higher TMB often correlates with a more favorable response to immunotherapy, as increased mutations result in more neoantigens, making the tumor more recognizable to the immune system. It is currently under investigation in multiple clinical trials for its potentially predictive and prognostic value in various cancers. It remains crucial to standardize TMB assessment methods to enhance its clinical utility. The GeparNuevo study showed that within the durvalumab group, patients with a higher TMB exhibited significantly higher rates of pathological complete response (pCR) than those with a lower TMB.19 The Keynote-119 study showed that mTNBC patients with a TMB of ≥10 mut/Mb benefited from pembrolizumab monotherapy.20 However, the Keynote-086 study did not identify a correlation between TMB and pembrolizumab efficacy.21 Although the National Comprehensive Cancer Network (NCCN) guidelines recommend pembrolizumab for advanced breast cancer patients with high TMB (≥10 mutations/Mb), there is still controversy over the use of TMB as a predictive marker for the efficacy of ICIs. This controversy stems from the lack of a unified threshold for determining TMB levels and the potential development of resistance to immunotherapy in patients with high TMB in certain situations. Therefore, more research is needed to explore the relationship between TMB and the efficacy of ICIs.
Stromal tumour-infiltrating lymphocytes (sTILs)
sTILs are white blood cells that have migrated into tumor tissue and represent the host’s immune response against cancer cells.22 Their presence in tumor tissues varies across different cancer types but is particularly significant in certain cancers, such as melanoma and breast cancer. High levels of sTILs often correlate with improved clinical outcomes, making it a valuable prognostic biomarker. Several studies have shown that the levels of sTILs correlate with the efficacy of immunotherapy.23,24 In the Keynote-173 study for early-stage TNBC, showed that patients with higher pre-treatment sTIL levels demonstrated elevated pCR rates.25 Similarly, the Keynote-086 study of mTNBC revealed a positive correlation between the efficacy of the monoclonal antibody pembrolizumab and sTIL levels.26 The Impassion130 study demonstrated that patients with sTILs ≥10% benefitted from the combination of atezolizumab and chemotherapy.17 In the GeparNuevo study, baseline sTIL levels were independent predictors of pCR but could not predict the efficacy of the monoclonal antibody durvalumab.27 Overall, sTILs exhibit a predictive value for treatment efficacy, but more prospective data from larger sample sizes are necessary to confirm their significance.
CD8-positive T cells
CD8-positive T cells, also known as cytotoxic T cells, are essential components of the immune system and play a crucial role in protecting the body against viruses and tumors.28 These cells identify and directly target infected or malignant cells by recognizing specific antigens present on these cells. Upon detection, they release cytotoxic agents that induce cell death. The ability of these cells to target and kill aberrant cells makes them integral to adaptive immunity. In the study involving the monoclonal antibody atezolizumab, baseline levels of CD8-positive T cell counts correlated with both progression-free survival (PFS) and the overall survival (OS) period.29 However, no correlations were observed between their baseline levels and treatment efficacy in a phase Ib study that used paclitaxel-albumin to treat mTNBC.30 Notably, the Impassion 130 study indicated that only patients who were CD8-positive and PD-L1-positive benefited from the treatment.17 Hence, the predictive efficacy of CD8-positive T cells needs further exploration.
The NCCN has issued guidelines for breast cancer that recommend PD-L1 expression as a criterion for choosing pembrolizumab treatment for patients with advanced breast cancer. However, when neoadjuvant treatment is administered, PD-L1 expression should not be considered when choosing a treatment plan. There are still some unresolved issues with predictive markers for efficacy, and with the support of new technologies such as single-cell sequencing and artificial intelligence, more exploration of these markers is expected.
Main clinical research progress of ICIs combined with chemotherapy
Neoadjuvant treatment
Neoadjuvant treatment for breast cancer represents a pre-surgery therapeutic approach aimed at reducing the size and extent of the cancer.31 This strategy, frequently involving chemotherapy, hormone therapy, or targeted drugs, enhances the effectiveness of subsequent surgery by minimizing the likelihood of residual disease. The benefits include increased rates of breast conservation, and a higher probability of pCR, translating into improved survival rates. Moreover, it allows for the rapid assessment of therapeutic efficacy. Follow-up studies are crucial for monitoring potential recurrence or progression. Despite potential adverse effects, the neoadjuvant approach is critical in personalized cancer management. Since PD-1 monoclonal antibodies in combination with chemotherapy significantly prolong the PFS and OS of mTNBC patients with PD-L1 expression ≥1%, reflecting their positive effects, researchers are now exploring the effects of ICIs on the efficacy and survival of TNBC patients in the neoadjuvant treatment setting.
The Keynote522 study showed that the addition of pembrolizumab to chemotherapy before surgery, followed by nine cycles of pembrolizumab maintenance therapy after surgery, increased the pCR rate by 14% and the 3-year event-free survival (EFS) rate by 7.7%.32 The pCR rates for PD-L1 positive patients who received chemotherapy +/- pembrolizumab were 68.9% and 54.9%, while for PD-L1 negative patients were 45.3% and 30.3%, respectively. The combination therapy with pembrolizumab significantly increased the pCR rate, and reduced the number of events, regardless of PD-L1 status. In terms of safety, the incidence of immune-related adverse events significantly increased in the pembrolizumab and chemotherapy group. The common adverse events were consistent with the usual spectrum of adverse events associated with immunotherapy. However, two deaths (0.3%) due to immune adverse events were observed in the combination treatment group. Based on this study, the NCCN Breast Cancer Guidelines recommend pembrolizumab in combination with carboplatin and paclitaxel, followed by cyclophosphamide and either doxorubicin or epirubicin for high-risk TNBC neoadjuvant treatment. The 2022 CSCO Breast Cancer Guide also includes chemotherapy combined with PD-1 inhibitors as a III-level recommendation for TNBC neoadjuvant treatment.
Atezolizumab has also been the subject of numerous studies exploring its efficacy in TNBC neoadjuvant treatment. The Impassion031 study investigated neoadjuvant treatment with paclitaxel-albumin followed by doxorubicin combined with cyclophosphamide +/- atezolizumab, with pCR rates of 58% and 41%, respectively.33 Regardless of PD-L1 status, the combination with atezolizumab increased the pCR rate in patients. However, in the NeoTRIP study, the improvement in pCR rates after adding atezolizumab was not statistically significant.34 In the GeparNuevo study, patients receiving durvalumab in combination with paclitaxel-albumin, epirubicin, and cyclophosphamide had a 9% increase in the pCR rate, but the difference was not statistically significant.
Thus, different ICIs, different combinations of chemotherapy regimens, and different stages among the participants can all affect the efficacy of adding ICIs. Differences in the mechanisms of action between PD-1 and PD-L1 monoclonal antibodies may also affect the final treatment efficacy. Further exploration of the study populations, plans, and predictive markers, is necessary to provide a clinical basis for the application of ICIs in breast cancer treatment.
Paclitaxel albumin
Paclitaxel albumin commonly known as Abraxane, is a novel, protein-bound particle form of the traditional chemotherapy drug.35,36 By utilizing albumin, a natural protein, Abraxane eliminates the need for chemical solvents, thereby reducing the risk of adverse events in patients. Enhanced solubility and transportation efficiency, along with increased effectiveness, make this medication a preferred choice for metastatic breast cancer treatment. Clinical trials have shown that, compared to standard paclitaxel, paclitaxel albumin significantly increases the response rate and improves survival time in metastatic breast cancer patients. Therefore, paclitaxel albumin contributes significantly to the advancement of breast cancer treatment options. The Impassion130 study in 2018 was the first to confirm that the combination of atezolizumab and paclitaxel albumin as a first-line treatment for mTNBC extended PFS by 2.5 months and OS by 7 months in both the intention-to-treat population (ITT) and patients with PD-L1 expression ≥1%.37 Based on this, the combination of atezolizumab and paclitaxel albumin was approved for first-line treatment for PD-L1 ≥1% mTNBC. However, the IMpassion 131 study evaluating the efficacy of paclitaxel +/- atezolizumab for the treatment of unresectable, locally advanced, or metastatic TNBC did not improve PFS or OS in either the ITT population or patients with PD-L1 ≥1%. As a result, the U.S. Food and Drug Administration (FDA) has withdrawn its approval for the use of atezolizumab in the treatment of mTNBC.
The Keynote355 study showed that chemotherapy alone or in combination with pembrolizumab as a first-line treatment for patients with PD-L1 [combined positive score (CPS) ≥10] mTNBC resulted in a median PFS of 9.7 and 5.6 months respectively and a median OS of 23.3 and 16.1 months respectively.38 The combination with pembrolizumab significantly improved the PFS and OS of patients with PD-L1 (CPS scores ≥10). For patients with PD-L1 (CPS scores ≥1) mTNBC, the combination with pembrolizumab extended PFS by two months, but the difference in OS was not statistically significant.
The 2022 4th edition of the NCCN Breast Cancer Guidelines recommends pembrolizumab in combination with chemotherapy as a first-line treatment for PD-L1 CPS (22C3) ≥10 mTNBC patients.
Combined ICIs and targeted treatment
ICIs combined with antibody-drug conjugates (ADCs)
ADCs represent a critical advancement in breast cancer treatment.39 Mechanistically, ADCs may enhance anti-tumor efficacy by inducing the infiltration of CD8+ T cells into tumors and increasing the sensitivity of tumors to immune checkpoint inhibitor therapy.40 Designed to deliver cytotoxic drugs specifically to cancer cells, ADCs spare healthy tissues, minimizing overall toxicity. ADCs consist of a monoclonal antibody linked to a potent drug via a biodegradable linker. Datopotamab deruxtecan (Dato-DXd) is an ADC drug targeting human trophoblast cell-surface antigen 2, with its conjugated chemotherapy drug being a topoisomerase I inhibitor.41 Preliminary results from the Phase Ib/II BEGONIA study were announced at the 2022 ESMO Breast Cancer, which explored the efficacy and safety of Dato-DXd combined with durvalumab monotherapy in the first-line treatment of mTNBC. The study included 29 patients for analysis, with an overall response rate (ORR) of 74%. Two patients achieved complete remission, and the treatment safety profile was favorable. We anticipate large-scale Phase III clinical trials to further confirm the efficacy of Dato-DXd in mTNBC patients.42
ICIs combined with poly ADP-ribose polymerase inhibitors (PARPis)
Breast cancers with the BRCA 1/2 mutations have increased immunogenicity.43 PARPis are a class of drugs that have revolutionized the treatment of breast cancer, particularly those associated with BRCA mutations.44 They obstruct DNA repair pathways in cancer cells, thereby enhancing the efficacy of chemical and radiation therapies. With this mechanism, PARPis selectively target cancer cells while leaving healthy cells virtually unscathed. Clinically approved PARPis, such as olaparib and talazoparib, have shown significant promise in improving PFS in breast cancer patients. Additionally, ongoing research aims to broaden the utilization of these agents, enhance their efficacy, and manage resistance. The TOPACIO/KEYNOTE-162 trial showed that in the treatment of second-line and later TNBC, pembrolizumab combined with niraparib had an ORR of 21% and a disease control rate of 49%.45 Subgroup analysis revealed that patients with breast cancer susceptibility gene (BRCA) mutations had a significantly increased ORR compared to those with the wild type (47% versus 11%). The ORR reached 32% in patients who were PD-L1 positive, a significantly higher percentage compared to the 8% ORR in PD-L1 negative patients.46
In neoadjuvant studies, the I-SPY2 study compared the efficacy of durvalumab monotherapy + olaparib and paclitaxel to paclitaxel alone as a neoadjuvant treatment for HER-2 negative breast cancer patients. In the TNBC subgroup, the pCR rate was 47% in the combination treatment group and 27% in the paclitaxel alone group. Therefore, many studies are currently underway exploring the efficacy of PARPis combined with ICIs in neoadjuvant and advanced stages.47,48
ICIs combined with small molecule anti-angiogenic inhibitors
Small molecule anti-angiogenic inhibitors present a novel approach to combating breast cancer.49 The defining characteristic of these inhibitors lies in their ability to stall angiogenesis, the process by which cancers form new blood vessels to feed their rapid growth. By limiting angiogenesis, these inhibitors effectively starve tumors, impeding their development. Several inhibitors, including sunitinib and sorafenib, have shown promising results in clinical trials for breast cancer treatment.50–52 Additionally, anti-vascular agents may target vascular endothelial growth factor receptor, thereby inhibiting neovascularization and promoting normalization of tumor vasculature, which may recruit more immune cells, reverse the suppressed immune microenvironment and sensitize tumors to immunotherapy. The minimal toxicity of these agents has been instrumental in reducing adverse effects. Despite these challenges, the development of small molecule anti-angiogenic inhibitors for breast cancer treatment remains a promising field of study.
Several small sample size phase II clinical trials conducted by Chinese researchers exploring the treatment of mTNBC with small molecule anti-angiogenic inhibitors combined with PD-1 monoclonal antibodies have shown promising progress. The ORR for apatinib combined with camrelizumab was 43.3%, with a median PFS of 3.7 months.53 Based on more accurate molecular typing, the Fudan University Cancer Hospital’s FUTURE-C-plus study for mTNBC of immunoregulatory type, which used famitinib combined with camrelizumab + paclitaxel-albumin treatment, showed an ORR of 81.3%, a median PFS of 13.6 months, and a durable response period of 14.9 months.54
ICIs combined with other small molecule inhibitors
A phase II study exploring the efficacy and safety of cabozantinib combined with nivolumab in the treatment of mTNBC reported that only 1/18 (6%) of patients achieved ORR, causing the trial to be terminated early.55
Overactivation of mitogen-activated protein kinase (MAPK) in breast cancer is also associated with resistance to immunotherapy. In the COLET study, cobimetinib, a MAPK inhibitor, was investigated in combination with chemotherapy ± atezolizumab as a first-line treatment for mTNBC. The cobimetinib/paclitaxel ORR was 38.3%, and the placebo/paclitaxel ORR was 20.9%. The ORR for cobimetinib + atezolizumab + paclitaxel and albumin-bound paclitaxel was 34.4% and 29.0%, respectively, suggesting that these combination strategies are promising.56,57
Conclusion
Compared to patients with HER-2 positive and hormone receptor positive breast cancer, TNBC patients lack effective treatment targets. Exploration of the tumor microenvironment has revealed that TNBC may have increased immunogenicity. Recent clinical research advancements have led to the introduction of novel treatment strategies for TNBC patients. ICIs combined with chemotherapy have demonstrated improved PFS in PD-L1-positive mTNBC patients. Additionally, this combination has shown the potential to increase the pCR rate in neoadjuvant treatment. The combination of ICIs with PARPis, anti-angiogenesis inhibitors, or MAPK inhibitors may be effective for treating mTNBC. More precise molecular subtyping provides the basis for more targeted treatments. As research progresses, we anticipate an increased application of more targeted treatments, immunotherapies, and different drug combinations in the management of TNBC.
Abbreviations
- Dato-DXd:
datopotamab deruxtecan
- ADCs:
Antibody-Drug Conjugates
- HER-2:
human epidermal growth factor-2
- ICIs:
immune checkpoint inhibitors
- MAPK:
mitogen-activated protein kinase
- NCCN:
National Comprehensive Cancer Network
- ORR:
overall response rate
- PARPi:
Poly ADP-ribose polymerase inhibitors
- PD-1:
programmed death receptor-1
- PD-L1:
programmed death ligand-1
- PFS:
progression-free survival
- TMB:
tumor mutational burden
- TNBC:
triple-negative breast cancer
- mTNBC:
metastatic TNBC
- pCR:
pathological complete response
- sTILs:
stromal tumor-infiltrating lymphocytes
Declarations
Acknowledgement
None.
Funding
This study is supported by the Science and Technology Commission of Shanghai Municipality (22Y11912800) and the Natural Science Foundation of Shanghai (20ZR1412400).
Conflict of interest
The authors have no conflicts of interest related to this publication.
Authors’ contributions
XYS and LF: Study concept, and drafting and editing of the manuscript. All authors revised the manuscript critically and approved the version to be published.

 Author information
Author information