Introduction
Adverse drug reactions (ADRs) present a significant challenge in clinical settings, with a notably higher incidence among hospitalized patients compared to outpatients. Antibiotics, particularly beta-lactam (BL) antibiotics, are a common cause of these reactions.1,2 BL antibiotics, penicillins are frequently implicated due to their wide use and relatively low toxicity, despite being commonly associated with allergic responses.
Reports indicate that between 5% and 10% of hospitalized patients claim allergies to antibiotics, predominantly to BLs.1,2 This prevalence is notably high in specific cohorts, such as hematology patients (14%), influencing antibiotic prescribing practices and correlating with increased readmission rates.3 However, comprehensive allergy evaluations often reveal a significantly lower true prevalence of BL allergies, barely exceeding 0.01%.
In primary care settings, the incidence of penicillin allergy labels is lower than in hospitalized patients but still substantially impacts clinical decisions. For instance, a study in the Netherlands identified that 0.6% of patients with a penicillin allergy label had increased usage of second-line antibiotics and higher healthcare utilization.4
The issue is more pronounced in the pediatric population. In North America and Europe, 5% to 8% of children are incorrectly labeled as allergic to Penicillin. Allergy studies show that over 94% of these children can tolerate Penicillin upon re-exposure.5
The emergence of bacterial resistance to antimicrobials has escalated into a major public health concern in the 21st century. Studies from the United Kingdom project that antimicrobial resistance (AMR) could result in 10 million annual deaths by 2050.6,7
Furthermore, a history of penicillin allergy is linked to an increased risk of acquiring multidrug-resistant organisms, including methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, and vancomycin-resistant Enterococcus.8–10
In 2021, the World Health Organization (WHO) described AMR as a “silent pandemic”, necessitating immediate global action.11–15 Projections suggest up to 350 million deaths attributable to AMR by 2050, with an annual death toll of 10 million according to United Nations reports.16,17
Despite significant investments in antibiotic research and development by public, private, and NGO sectors,18 the introduction of new antibiotics is insufficient to curb the AMR threat. Most newly approved antibiotics lack innovative mechanisms or chemical classes.19 This underscores the WHO’s emphasis on accurately identifying ADRs to BLs.20 Spain’s multidisciplinary approach to optimizing antibiotic use, especially for patients with adverse reactions to BLs, exemplifies national efforts in addressing this challenge, which will be discussed in this article.21
In conclusion, in most cases of adverse reactions to BL treatments, a standardized allergy study provides patients and their doctors with at least two outcomes:
De-labeling in most cases, with subsequent permission for future use;
The few patients truly “labeled” as allergic to BLs are not necessarily allergic to all other BLs (Cephalosporins, Carbapenems, and Monobactams).
The BL family: classification of BL antibiotics
The concept of antibiotics has evolved from being seen as merely small molecular weight substances produced by microorganisms to inhibit competitors, to now being recognized as signaling molecules within microbial ecosystems.22 This shift in understanding underscores the complexity of antibiotic actions and interactions in natural environments.
The groundbreaking discovery of penicillin’s antibacterial properties by Alexander Fleming in 1928 marked the onset of the BL antibiotics era. Penicillin, derived from the fungus Penicillium notatum, was observed to inhibit Staphylococcus aureus growth, with its chemical structure elucidated through X-ray crystallography in 1949.22
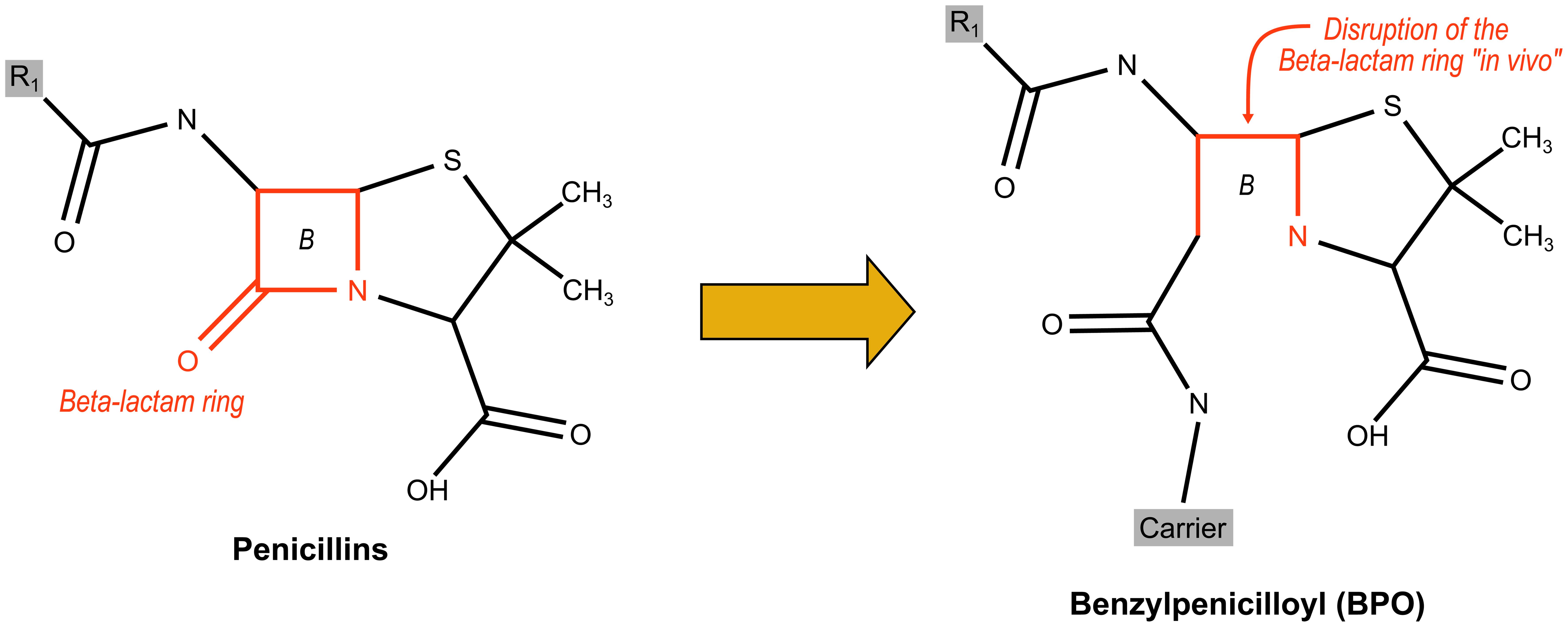
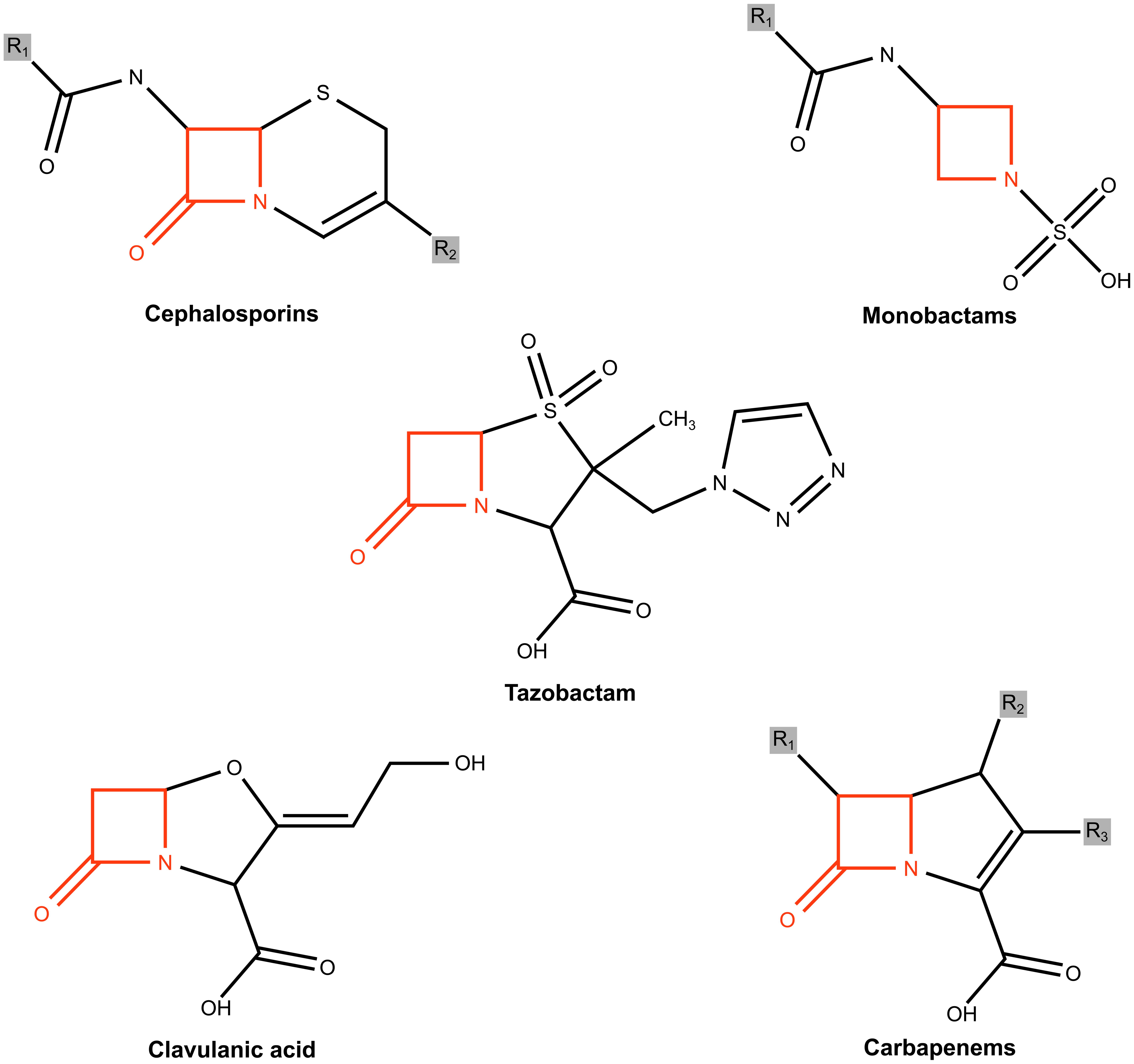
The BL family comprises both natural and semisynthetic antibiotics, unified by a characteristic four-membered BL ring crucial for inhibiting bacterial cell wall synthesis. Broadly, BL antibiotics can be categorized into four primary classes based on their cyclic structures: penams (penicillins), cephems (cephalosporins), carbapenems, and monobactams. Figure 1 illustrates the BL ring, while Figure 2 displays the diverse structures within the BL family.
Penams (penicillins)
Penams, which include penicillins, are distinguished by their reactive BL ring. This reactivity makes them susceptible to degradation both in vivo (where the BL ring tends to break and bind to plasma proteins) and by bacterial enzymes like beta-lactamases. To counteract this, inhibitors like clavulanic acid, tazobactam, and sulbactam have been developed. These inhibitors, shown in Figure 2, can irreversibly bind to beta-lactamases, protecting the antibiotic from enzymatic inactivation.
Cephems (cephalosporins)
Cephalosporins, derived from Cephalosporium acremonium and first isolated in 1954, constitute a major subgroup of cephems. Known for their efficacy and low toxicity, cephalosporins share a mechanism of action with penicillins, targeting peptidoglycan synthetases.
Despite their broad use, certain cephalosporins are susceptible to hydrolysis by beta-lactamases. They are classified into generations (first to fifth) based on their antibacterial spectrum.
Carbapenems
Carbapenems, including notable drugs like imipenem and meropenem, are effective against a wide range of organisms, including gram-negative bacilli, gram-positive bacteria, and anaerobes. Their pharmacological properties and antimicrobial spectrum are similar, making them valuable in treating various infections.
Monobactams
Unique for their monocyclic BL structure, monobactams exhibit resistance to beta-lactamases. They are particularly effective against gram-negative bacilli but lack activity against gram-positive bacteria or anaerobes.
Penicillin allergy: a paradigm in drug allergy research
Penicillin allergy research has been pivotal in drug allergy studies, with in-depth investigations leading to comprehensive understanding of penicillin’s active metabolites, which are widely utilized in both in vivo and in vitro diagnostics.
Immunological Characterization of BLs
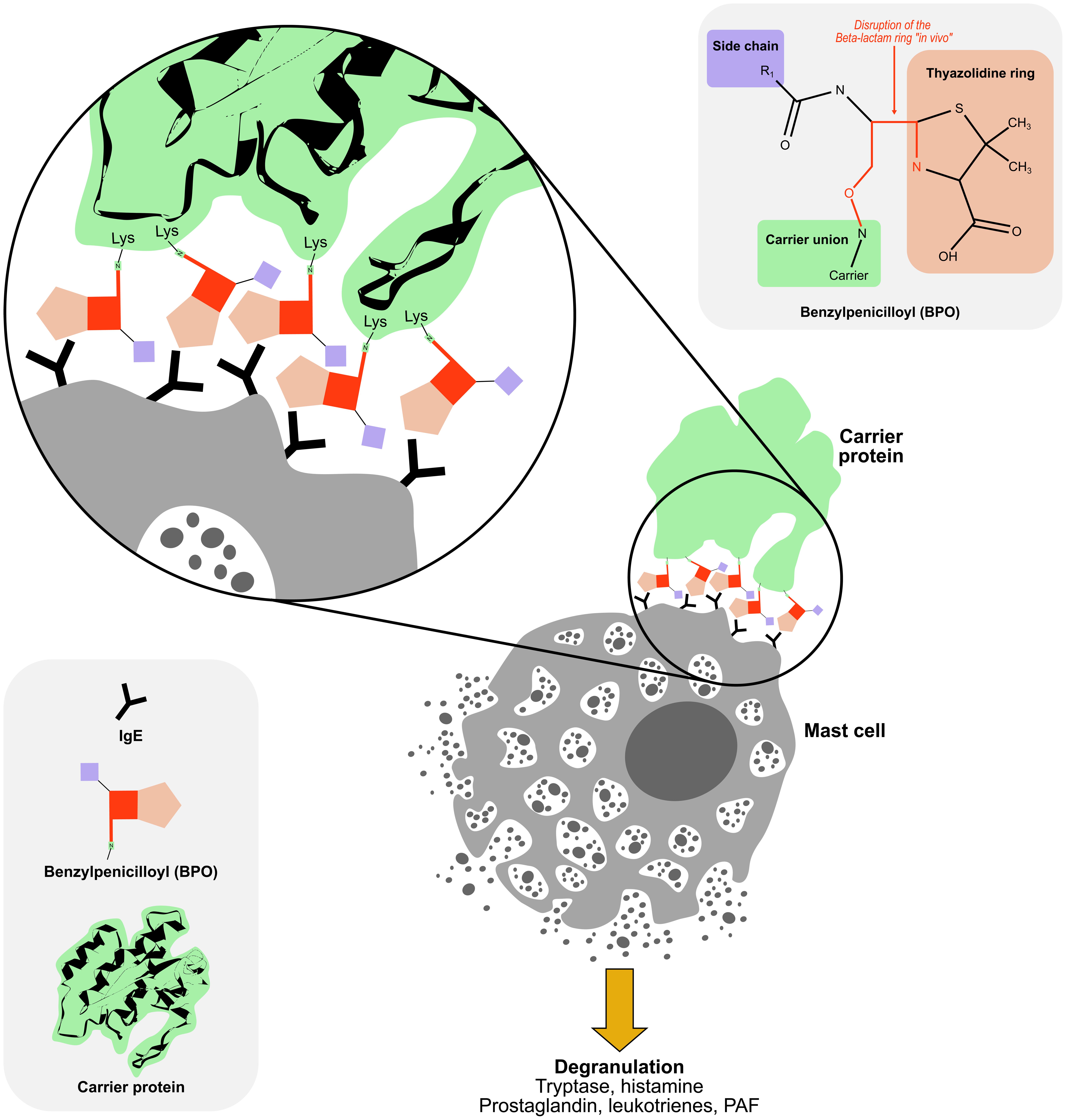
BLs, including penicillins, cephalosporins, aztreonam, and imipenem, are categorized immunologically as haptens. These low molecular weight structures require binding to transport molecules to form complete antigens and achieve full immunogenicity (see Fig. 3). This process entails the spontaneous opening of the BL ring, allowing these antibiotics to bind to amino groups of autologous proteins. This binding triggers a conformational change, leading the immune system to recognize these complexes as foreign entities.23 The resulting hapten-protein conjugates are processed by antigen-presenting cells, inducing the production of specific IgE antibodies against penicillin.
In the upper right part, benzylpenicilloyl (BPO) and the binding site of penicillin to IgE antibodies are represented according to De Haan’s studies with monoclonal antibodies. IgE-mediated reactions occur due to drugs acting as haptens, needing to bind to macro-molecules and form adducts that interact with IgE bound to a high-affinity receptor (FcεRI) on the surface of effector cells, mast cells and basophils. Activation and degranulation of mast cells and basophils are triggered, leading to the release of preformed inflammatory mediators, as well as the synthesis and secretion of lipid mediators and cytokines.
Upon re-exposure, these IgE molecules cross-link with high-affinity receptors (FcεRI) on effector cells (mast cells and basophils), triggering cell activation and degranulation. This cascade releases inflammatory mediators and stimulates the synthesis and secretion of lipid mediators and cytokines. Diagnostic identification of this IgE-mediated response primarily utilizes skin testing (ST) and quantification of specific serum IgE against penicillin, with serum tryptase measurements providing additional insights during the reaction.24
Penicillin Degradation Pathways
The immunochemistry of penicillins serves as a model for studying other drugs.
Penicillins undergo spontaneous BL ring rupture in vivo, leading to the formation of amide bonds with lysine residues on nearby proteins (Fig. 3). This process generates the major antigenic determinant, benzylpenicilloyl (BPO), which constitutes approximately 95% of the antigenic profile of penicillins.25
BPO, linked to a polylysine molecule, forms benzyl-penicilloyl-polylysine (PPL), a key component in routine ST commercially available by Diater® Spain.
The remaining 5% of penicillins follow alternative degradation pathways, resulting in various minor determinants capable of eliciting IgE-mediated responses. The commercially available Minor Determinant Mixture (MDM) includes benzylpenicillin and its hydrolysis products, benzylpenicilloate, and benzylpeniloate, underscoring the complexity of penicillin’s immunogenic profile.
Regarding the degradation pathways of other groups of BLs, while similar routes have been proven for some cephalosporins, we cannot affirm that they are identical to penicillin itself.
Antigenic determinants of penicillins
Research has highlighted the BPO group as a critical antigenic determinant in penicillins, with variations in the chemical structure of the side chain significantly influencing antibody recognition.26–28 The production of monoclonal antibodies against penicillins has revealed at least three antigenic determinants: the thiazolidine ring, the side chain, and a new determinant formed in vivo where the BL ring binds to proteins (See Fig. 3).29
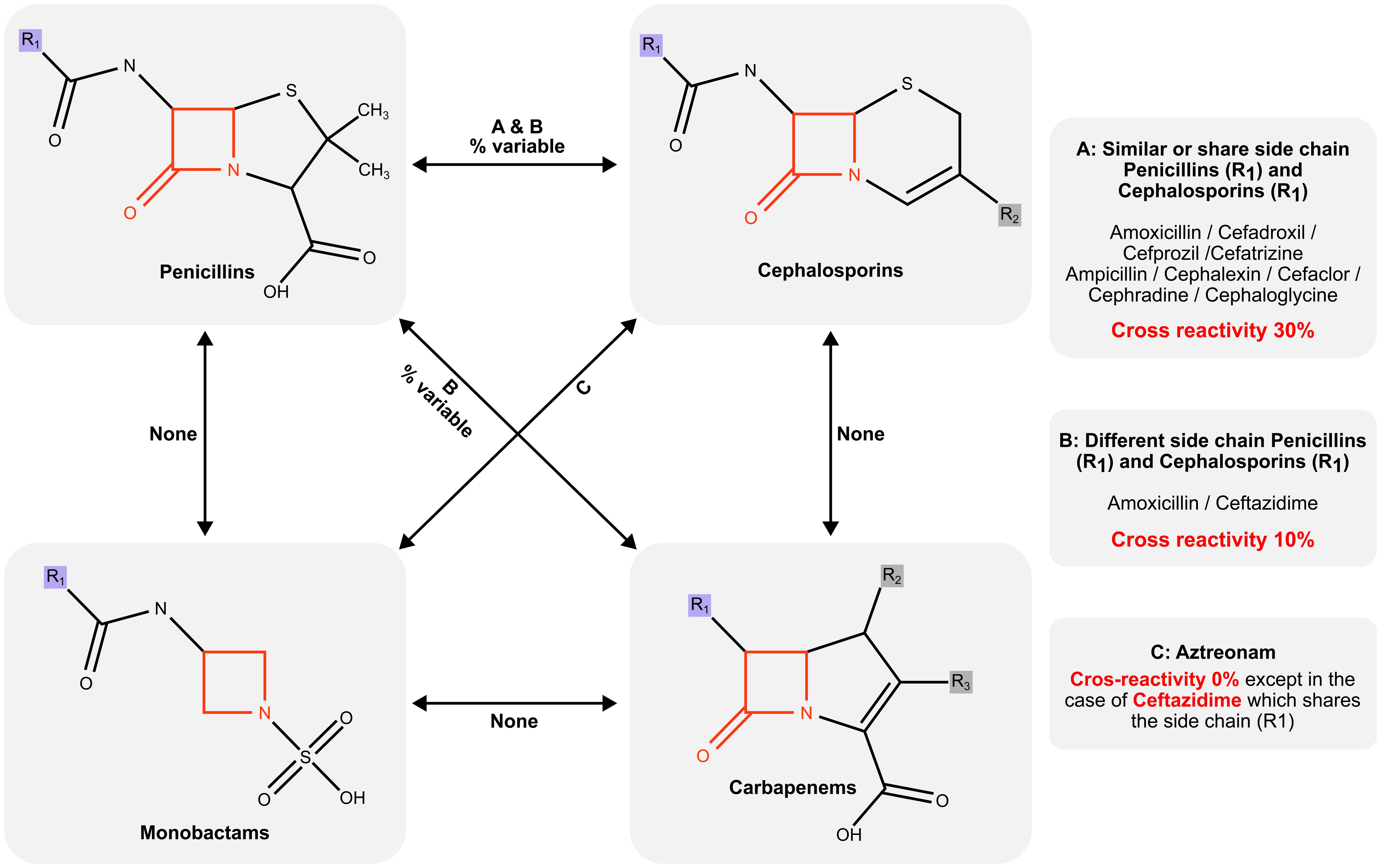
The side chain is pivotal in selective allergy, allowing for cross-recognition among penicillins, cephalosporins, monobactams, and carbapenems.30,31 Moreover, the newly identified determinant, formed by the union of the BL ring’s carbonyl group with a protein’s amino group, is common to all BLs and contributes to cross-reactivity. In cephalosporins, key IgE-binding sites include the side chain and the junction between the open cephem nucleus and the protein, as confirmed by monoclonal antibody studies with cephalexin.32
This complexity underlines that allergic responses are not solely attributable to the BL ring but also to the side chains, which can be identical across different BL groups, influencing cross-reactivity. The beta-lactam ring is just one contributing factor. It is important to note that different groups of BLs can contain identical side chains, as occurs between amoxicillin and cefadroxil (penicillin and cephalosporin) or between aztreonam and ceftazidime (monobactam and cephalosporin). This fact may condition cross-reactivity between BL groups as represented in Figure 4.
Significance of the side chain in BL allergies
The emergence of new penicillin derivatives, particularly aminopenicillins, has brought attention to the role of the side chain in BL allergies. These selective reactions, encompassing both immediate and non-immediate types, involve IgE- and T-cell-mediated immune mechanisms. Notably, research in Spain has revealed distinct patterns in BL allergies compared to findings in other regions, particularly highlighting unique selective reactions to Amoxicillin (AX).33,34 Patients who experienced anaphylactic reactions to AX were found capable of tolerating Benzylpenicillin (BP), suggesting a nuanced role of the side chain in allergic responses.
The contribution of the side chain to cross-reactivity between penicillins and cephalosporins has been a subject of ongoing research since the 1990s in Spain and more recently for other groups.35–39 This phenomenon seems to be more prevalent in European and Australian populations than in the United States, possibly due to differences in BL consumption patterns and variations in access to specialized allergy studies. The delayed licensure of major and minor penicillin determinants (PPL and MDM Diater® Spain) by the Food and Drug Administration in the USA until 2010 may have also contributed to these regional differences.40
In simplifying this complex interaction, IgE antibodies targeting the nuclear portion of the BL ring are implicated in cross-reactivity across BL classes. In contrast, antibodies specific to the BL side chain are associated with selective allergies. This distinction is crucial for understanding the allergenic potential of different BLs (see Fig. 4).
Cephalosporins, following penicillins, are the BL antibiotics most associated with selective allergic reactions. Although initially evaluated in penicillin-allergic individuals, increasing reports of cephalosporin-induced allergies are emerging as their usage expands. Conversely, allergic reactions to carbapenems are less frequently reported, likely due to their restricted use in parenteral administration.39
Classification of allergic reactions to BLs
The classification of allergic reactions to penicillins and other BLs primarily relies on the underlying immunological mechanisms, predominantly mediated by IgE antibodies or T-cells. These reactions are categorized into immediate and non-immediate types, each presenting distinct clinical features and temporal profiles.
Immediate reactions
Immediate allergic reactions to BLs typically manifest within 60 m of drug administration. These reactions indicate specific IgE antibodies targeting the hapten-carrier conjugate. Common clinical presentations include:
Urticaria/Angioedema: The most frequent manifestation, accounting for approximately 72% of immediate reactions;
Anaphylaxis: Occurs in about 10% of cases;
Bronchial Asthma: Observed in around 5% of patients.
In the adult population, immediate reactions are the most common, with occurrence rates ranging from 0.01% to 10%. Conversely, these reactions are less prevalent among pediatric patients with rates varying between 0.01% and 10%.
Non-immediate reactions
Non-immediate reactions typically develop within 1–72 h post-drug administration. These reactions are characterized by a broad clinical spectrum, predominantly involving the skin in about 90% of cases. The severity of these reactions can vary, ranging from mild (the most common) to potentially serious conditions (fortunately rare), such as:
Pustular Exanthema;
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS);
Stevens-Johnson Syndrome;
Toxic Epidermal Necrolysis;
Organ-specific reactions (including but not limited to hematological abnormalities).
In children and adolescents, unlike in adults, the most frequent adverse reactions due to BLs are maculopapular eruptions, often coinciding with infectious processes.41–44
These reactions typically overlap with rashes caused by the infections themselves, necessitating differential diagnoses from conditions like exanthema subitum, infectious mononucleosis, and infectious urticaria.
Allergy study for BL allergies
The diagnostic process for suspected BL allergies encompasses a comprehensive evaluation, including the morphology and chronology of the reaction, previous reactions to the medication, treatments to mitigate the ADR, and any antibiotics tolerated post-reaction.45
Diagnostic approach
Risk factors to consider include the medication’s nature (e.g., intermittent or continuous administration), patient-specific factors (age, genetic characteristics, concomitant diseases like cystic fibrosis, EBV, and HIV infections), and occupational exposure.45
However, medical history alone is insufficient for differentiating allergic from non-allergic reactions, necessitating more definitive tests like ST, patch testing (PT), and drug provocation tests (DPT).46–49
ST
Skin prick tests (SPT) and intradermal tests (IDT) are indicated for the assessment of subjects with immediate reactions, whereas delayed-reading skin tests and/or patch tests assess non-immediate reactions.
Skin tests
Skin tests, including skin prick tests (SPT) and intradermal tests (IDT) are the most validated methods for allergy confirmation.45 In immediate reactions, ST should be performed after a time interval of 3–6 weeks from the reaction. Initial SPT is conducted at the highest non-irritating concentration, with IDTs following if negative and read at a 20-m interval. The procedure must be stopped when a positive result is found, which is considered when the diameter of the wheal is at least 3 mm larger compared to the negative control and is surrounded by erythema. The panel of reagents for evaluating hypersensitivity reactions (HSR) to BL by ST includes the classic penicillin reagents and the commercially available major and minor determinants: PPL and MDM (Diater®, Leganés, Spain) but are not available in all European countries. The sensitivity of these tests is estimated at 70% if major and minor determinants of penicillin (PPL and MDM), amoxicillin, and the suspected BL are used. The positive predictive value of skin tests has been estimated ranging from 40% to 100% in immediate reactions. In patients with non-immediate reactions, IDTs can be positive on delayed readings.
Patch tests (PT)
PT can be a valuable diagnostic tool, especially in certain forms of Severe Cutaneous Adverse Reactions (SCAR), despite its lower sensitivity but high specificity. PT is particularly useful in cases where multiple drugs have been used simultaneously, making it challenging to pinpoint the culprit drug. It can aid in identifying the specific drug responsible for the adverse reaction and guide future treatment decisions. Additionally, PT can help healthcare providers avoid reintroducing the offending drug, thus preventing the recurrence of SCAR. After the resolution of the reaction, patch tests should be performed as the first diagnostic method to identify the culprit drug(s).
PT is the method of choice in those individuals with contact dermatitis; they are useful in MPE, flexural exanthemas, and, if conducted in situ, also in fixed drug eruptions.PT is applied on the upper back according to the methods used for contact dermatitis. When negative, PT should be supplemented with IDT with delayed readings, which are more sensitive than PT. In subjects with fixed drug eruptions, PT should be applied to the site of eruption (residual pigmented lesion).
It is recommended to perform patch tests at least 4 weeks after the disappearance of cutaneous adverse drug reactions and discontinuation of systemic glucocorticoids or immunosuppressive drugs, as well as after ultraviolet exposure on the tested skin area. In DRESS, PT must be conducted at least 6 months after the disappearance of cutaneous reactions and after verifying the absence of reactivation of herpes group viruses (i.e., HHV6, HHV7, EBV, and human cytomegalovirus).
Patches are left for 48 h, then read at 72 h and/or 96 h, with positive reactions indicated by erythema, skin infiltration, or vesicle formation.45 In some European countries, a series of 11 drugs diluted at 10% in petrolatum is available and marketed by Chemotechnique (Velinge). When the active ingredient is available in pure form (e.g., lyophilized), it is recommended to dilute it at 10% in petrolatum.45
In vitro tests
In vitro testing currently does not provide sufficient clinical utility to support its use in de-labeling.50 Both the specific IgE determination and the basophil activation test lack sufficient sensitivity and predictive value to guarantee safe de-labeling. In addition, the basophil activation test, a flow cytometry-based test that measures drug-induced basophil activation by examining alterations in the expression of basophil markers such as CD63 and CD203c, is not available in many centers.51,52
Multidisciplinary approach and risk stratification
A multidisciplinary approach involving allergists, infectious disease specialists, and pharmacists, is recommended for managing these patients (Table 1).21,53 This approach involves risk stratification into three categories:
Risk stratification in the de-labeling of adverse drug reactions to beta-lactams
| Non-immune-mediated adverse drug reaction | Low-risk patients | High-risk patients |
|---|---|---|
| Isolated gastrointestinal symptoms; Mucocutaneous candidiasis or headache as the sole symptom; Family history of antibiotic allergy in the absence of exposure or symptoms after exposure; Rash in the absence of exposure or culprit tolerated after the reaction. | Mild and moderate maculopapular rash in children; Mild maculopapular rash in adults; Other rashes: fixed drug eruption, contact dermatitis, or palmar exfoliative exanthema; Isolated generalized pruritus; Local infiltrated reaction to intramuscular administration in the absence of haematoma; Unknown reaction without mucosal involvement, skin desquamation, organ involvement, or presyncope. | Type I immediate reactions: Upper and/or lower respiratory symptoms; Urticaria; Bronchospasm; Angioedema; Collapse; Poorly described symptoms in patients with significant cardiovascular comorbidity; Need of epinephrine or hospital care during the alleged episode of allergy; Kounis syndrome.53 Type II-IV delayed reactions: Moderate-severe maculopapular rash in adults; Desquamative maculopapular exanthema with or without mucosal involvement (SJS, TEN); Drug reaction with eosinophilia and systemic symptoms (DRESS); Systemic vasculitis/Serum-sickness– like reaction; Specific organ reactions (i.e. acute interstitial nephritis); Haemolytic anaemia; Need of hospital care during the alleged episode of allergy; Acute generalized exanthematous pustulosis. |
Low-risk: Patients who tolerated the BL post-ADR or presented with nonspecific symptoms (e.g., nausea, vomiting, diarrhoea, headache, paraesthesia) do not require extensive study;
High-risk Immediate Type I Reactions: Patients with anaphylaxis, bronchospasm, angioedema, laryngeal oedema, or hypotension;
High-risk Non-immediate Type II-IV Reactions: Severe HSR, such as Stevens-Johnson Syndrome, toxic epidermal necrolysis, acute interstitial nephritis, DRESS, and haemolytic anaemia.
DPT
DPT is considered the gold standard in allergy diagnosis, aiming to establish a definitive diagnosis and assess cross-reactivity with related drugs. DPT involves the controlled administration of the drug in question, with varying protocols based on the nature of the initial reaction. It’s imperative that DPT be performed under medical supervision, with preparedness for immediate treatment of any adverse responses.
Various recent literature reviews and meta-analyses have shown that more than 90% of patients with a history of ADR to penicillins undergoing treatments with BLs tolerate them without prior study.51,52,54,55 However, those of us who face true allergic reactions daily know that the risk cannot be assumed blindly. In this sense, various authors propose an interdisciplinary approach to risk assessment, because there is a need for validated approaches that optimally combine the use of medical history and oral challenge tests with or without prior ST.45
The goal of DPT is to establish a firm diagnosis and confirm the absence of cross-reactivity with related drugs to prescribe alternative drugs. Severe index reactions (severe cutaneous syndromes, vasculitis, severe anaphylaxis…), some patient comorbidities (poorly controlled asthma, pregnancy…), and the presence of positive skin tests with a drug are contraindications for carrying out DPT. Taking all this into account, in most cases, it is important to carry out DPT in a drug allergy study to avoid less effective, more toxic, and more expensive alternative treatments; especially in patients who have suffered mild reactions.53,49 Taking all these into account, in drug allergy studies it is important to carry out graded DPT to avoid less effective, more toxic, and more expensive alternative treatments in most cases.49,51 Furthermore, a multicenter study carried out by the Spanish Society of Allergy and Clinical Immunology (SEAIC) confirms that completing a drug allergy study with DPT improves the quality of life of patients who have experienced anaphylaxis.56
Graded DPT consists of the controlled administration of progressively increasing doses of a drug, generally at intervals of 15–30 m. It can be oral, subcutaneous or intravenous and can be performed with the culprit drug or with alternative drugs. Before commencing the test, the patient must sign an informed consent. During the test, vital signs and both subjective and objective symptoms should be monitored. To conclude, DPT should always be carried out by trained health personnel and with adequate facilities with access to adrenaline and other treatments.49
In the case of BL allergy, numerous studies have confirmed the safety and utility of DPT in both children and adults.44,57 Before performing a DPT, a risk assessment should be carried out taking into account characteristics related to the drug (e.g., route of administration and posology), the type of index reaction, and characteristics related to the patient such as age and concomitant diseases.45 With regard to the index reaction, immediate reactions must be differentiated from non-immediate reactions.58Table 1 shows a summary of risk stratification in BL allergy taking into account the index reaction, classifying patients as low-risk or high-risk.
Following the European Academy of Allergy & Clinical Immunology (EAACI) position paper, after obtaining a detailed medical history, in vitro and/or skin tests should be conducted. If the results are negative, DPT with the culprit drug can be considered.45 It should be noted that in recent years some authors have reported the possibility of performing DPT without prior skin tests in patients who have experienced mild non-immediate reactions such as maculopapular rash and urticaria, especially in children but also in adults.59–62
Various DPT protocols have been proposed depending on whether the index reaction is immediate or non-immediate. There is great heterogeneity in studies regarding the final dose, dose escalation intervals, and duration of the DPT.63 In non-immediate reactions, regarding the duration of the DPT, some authors consider that carrying it out in a single day is sufficient but others prefer a prolonged regimen over several days.43,49,64–67 In relation to this topic, the EAACI position paper recommends carrying out a one-day DPT.45
Finally, when BL allergy is confirmed, it is crucial to confirm tolerance to related drugs considering cross-reactivity. Many patients allergic to aminopenicillins selectively tolerate other BLs. Cross-reactivity between penicillins and cephalosporins varies from 10 to 30% depending on the similarity of the R1 side chain. Patients allergic to ceftazidime should avoid aztreonam because they share the same side chain.45,49 Taking all this into account, the EAACI position paper on the diagnosis of BL allergy proposes an algorithm for the use of alternative antibiotics in patients with suspected BL allergy, when an allergy study is not available (Table 2).45
EAACI position paper: Algorithmic approach to patients with histories of hypersensitivity reactions to specific penicillins and/or cephalosporins and an immediate need for antibiotic therapy when referral to an allergist is not feasible
| Non-immediate reactions to penicillins and/or cephalosporins high-risk subjects | Immediate reactions to penicillins and/or cephalosporins high-risk subjects | Non-immediate or immediate reactions to penicillins and/or cephalosporins low-risk subjects |
|---|---|---|
| Avoid penicillins and cephalosporin, and use non-BL-antibiotics by microbial coverage | Avoid using the entire class of the responsible BL | Use full-dose 3rd/4th/5th generation cephalosporins in subjects who reacted to penicillins or full-dose penicillins with side chains different from those of the responsible cephalosporins in subjects who reacted to cephalosporins |
| OR | OR | OR |
| Use carbapenems or aztreonam1 by graded challenge,2 after a careful risk-benefit analysis | Used by graded challenge 3rd/4th/5th generation cephalosporins in subjects who reacted to penicillins3 or penicillins with side chains different from those of the responsible cephalosporins in subjects who reacted to cephalosporins3 | Use full-dose carbapenems or aztreonam1 |
| OR | OR | OR |
| Use by graded challenge 3rd/4th/5th generation cephalosporins in subjects who reacted to penicilins2 or penicillins with side chains different from those of the responsible cephalosporins in subjects who reacted to cephalosporins,2 very carefully in SJS/TEN | Use carbapenems or aztreonam1 by graded challenge3 | Use non-BL antibiotics by microbial coverage |
| OR | ||
| Use non-BL antibiotics by microbial coverage |
Desensitization
In cases where the allergenic drug is necessary, and no alternatives are viable, rapid drug desensitization (RDD) may be employed. This technique involves gradually increasing drug doses to temporarily modify the patient’s immune response within hours.67
Through RDD, inhibition of mast cells and basophils is achieved, preventing the release of mediators and consequently the allergic reaction.68 RDD protocols are effective for mild type I and IV hypersensitivity reactions but are absolutely contraindicated in severe type II, III, and IV reactions such as SCAR.67 Like DPT, it is a high-risk procedure, so it must be performed by trained personnel in a safe environment.67 Various RDD protocols with different BL drugs, both oral and intravenous, have been found to be safe and effective.67,69
In conclusion, this procedure allows for the maintenance of first-line antibiotics, leading to greater efficacy, fewer side effects, and a longer life expectancy for patients compared to the use of second-line therapies.
Recommendations on empirical antibiotic use
The misuse of antibiotics, often due to mislabeled allergies, contributes to bacterial resistance. Therefore, updated guidelines on empirical antibiotic therapy are crucial. In cases of confirmed BL allergy, alternative antibiotics with minimal cross-reactivity should be considered. This approach aims to reduce the use of broad-spectrum antibiotics, thereby diminishing the risk of bacterial resistance and related complications.
Antibiotics, specifically BL, are some of the most prescribed drugs worldwide. They produce several predictable adverse reactions that are often falsely labeled as allergies, leading to the inappropriate promotion of other broad-spectrum antibiotics and contributing to the increase in bacterial resistance.51 More than 10% of the population is considered allergic to penicillin, the majority of whom allergy can be ruled out after performing an allergy study.70–72
To reduce inappropriate antibiotic use, it is recommended to consult updated guidelines on empirical antibiotic therapy. As regards bacterial resistance caused by using broad-spectrum antibiotics in BL-allergic patients, an increase in surgical infections, proliferation of multidrug-resistant bacteria such as methicillin-resistant Staphylococcus aureus, and a higher rate of Clostridium difficile infections, among others, have been observed. These reported problems caused longer hospitalizations and increased mortality in patients with an allergy label compared to those receiving BL.21,51
Suspected unconfirmed BL allergy also leads to the use of less effective, more expensive, and more toxic second-line antibiotics. For example, macrolides frequently cause gastrointestinal discomfort, rupture of the Achilles tendon due to quinolones, and ototoxicity secondary to aminoglycosides.45
In immediate BL allergy, the drugs that present the least cross-reactivity with penicillin are aztreonam (0%) and carbapenems (0.87%) which are considered safe in the majority of patients labeled with penicillin allergy.21 In delayed BL allergy, recommendations cannot be made on alternative drugs due to limited information regarding cross-reactivity between BLs in these cases. Each case must be assessed individually based on the results of complementary tests.
Various studies, including a Spanish multidisciplinary study summarizing the evidence, suggest that in some cases, hospitalized patients with moderate and severe infections with type I allergy to penicillin or cephalosporins, DPT could be performed with a BL that has low cross-reactivity with the culprit drug without needing to perform skin tests. Please refer to Figure 4 for more information.21
Conclusions
In conclusion, a comprehensive allergy study, including risk assessment, ST, DPTs, and, where necessary, RDD, is essential for accurate diagnosis and management of BL allergies. This approach not only ensures patient safety but also aids in preserving the efficacy of first-line antibiotics and mitigating the rise of antibiotic resistance.
Myth: A single adverse reaction to penicillin necessitates lifelong avoidance.
Reality: Most patients with a history of penicillin reactions are not truly allergic. Rigorous allergy testing and de-labeling can safely reintroduce penicillin in these cases.
Myth: Any adverse reaction to penicillin necessitates avoiding all beta-lactam antibiotics.
Reality: Not all beta-lactam allergies are cross-reactive. A thorough evaluation can identify safe beta-lactam options, avoiding unnecessary exclusion of effective antibiotics.
Comprehensive allergy testing is crucial to curtail the overuse of broad-spectrum antibiotics, thereby reducing bacterial resistance, hospitalization durations, and healthcare costs.
Implementing risk stratification prior to allergy testing can optimize the diagnostic process by eliminating unnecessary tests in low-risk groups, such as children with mild, nonspecific reactions, and expediting de-labeling in appropriate cases.
Understanding the role of the side chain in beta-lactam allergies provides nuanced guidance for urgent treatment in cases with suggestive histories, potentially allowing the use of alternative beta-lactams.
Standardized allergy studies are key to informed antibiotic selection, particularly in patients with true beta-lactam allergies, facilitating the safe use of various beta-lactam antibiotics.
In summary, refining the approach to beta-lactam allergy testing and de-labeling is essential for enhancing patient care, reducing the burden of antibiotic resistance, and ensuring the judicious use of antibiotics. Emphasizing the distinction between myths and realities in beta-lactam allergy management can significantly improve therapeutic decisions and patient outcomes.
Declarations
Acknowledgement
We thank DIATER for invaluable English language support and Maialen Mendiguchia for the graphic designs.
Funding
None.
Conflict of interest
None.
Authors’ contributions
Conceptualization: MTA, PO, MG; Data curation: MTA; Formal Analysis: MTA; Supervision: MTA; Validation: MTA; Writing – original draft: MTA, PO, MG; Writing—Review & Editing: MTA, PO, MG. Design and creation of all graphics: MMA. All authors engaged in a thorough review of the manuscript and have given their approval for the final version to be published.

 Author information
Author information