Introduction
Central serous chorioretinopathy (CSC) is primarily characterized by localized serous retinal detachment, and its pathological mechanism involves dilated choroidal vessels, increased permeability, and impairment of retinal pigment epithelial (RPE) barrier function.1–3 CSC primarily encompasses acute and chronic CSC, in addition to an uncommon type known as bullous retinal detachment. Acute CSC can be detected early by optical coherence tomography (OCT), which shows serous retinal detachment (SRD) in the macular region, accompanied by reflective deposits caused by fibrous exudation; meanwhile, fundus fluorescein angiography (FFA) shows “ink blot” or “smoke stack” hyperfluorescence.4,5 Chronic CSC often presents with more varied imaging features and typically needs to be differentiated from other choroidal hypertrophic spectrum diseases, choroidal neovascularization, and uveitis. Bullous retinal detachment, on the other hand, is mainly characterized by a widespread diffuse SRD. The diagnosis, monitoring, and treatment of CSC rely on various imaging techniques, primarily OCT, FFA, and indocyanine green angiography (ICGA). With the advances and iterative updates in ocular imaging technologies and equipment, multispectral imaging, en face OCT, and OCT angiography (OCTA) have made the analysis of choroidal and retinal vascular structure and morphology possible. The comprehensive application of these imaging techniques can provide a reliable basis for the treatment, follow-up, and identification of neovascular components in CSC. This article reviews the multimodal imaging features and the latest progress in CSC research (Fig. 1).
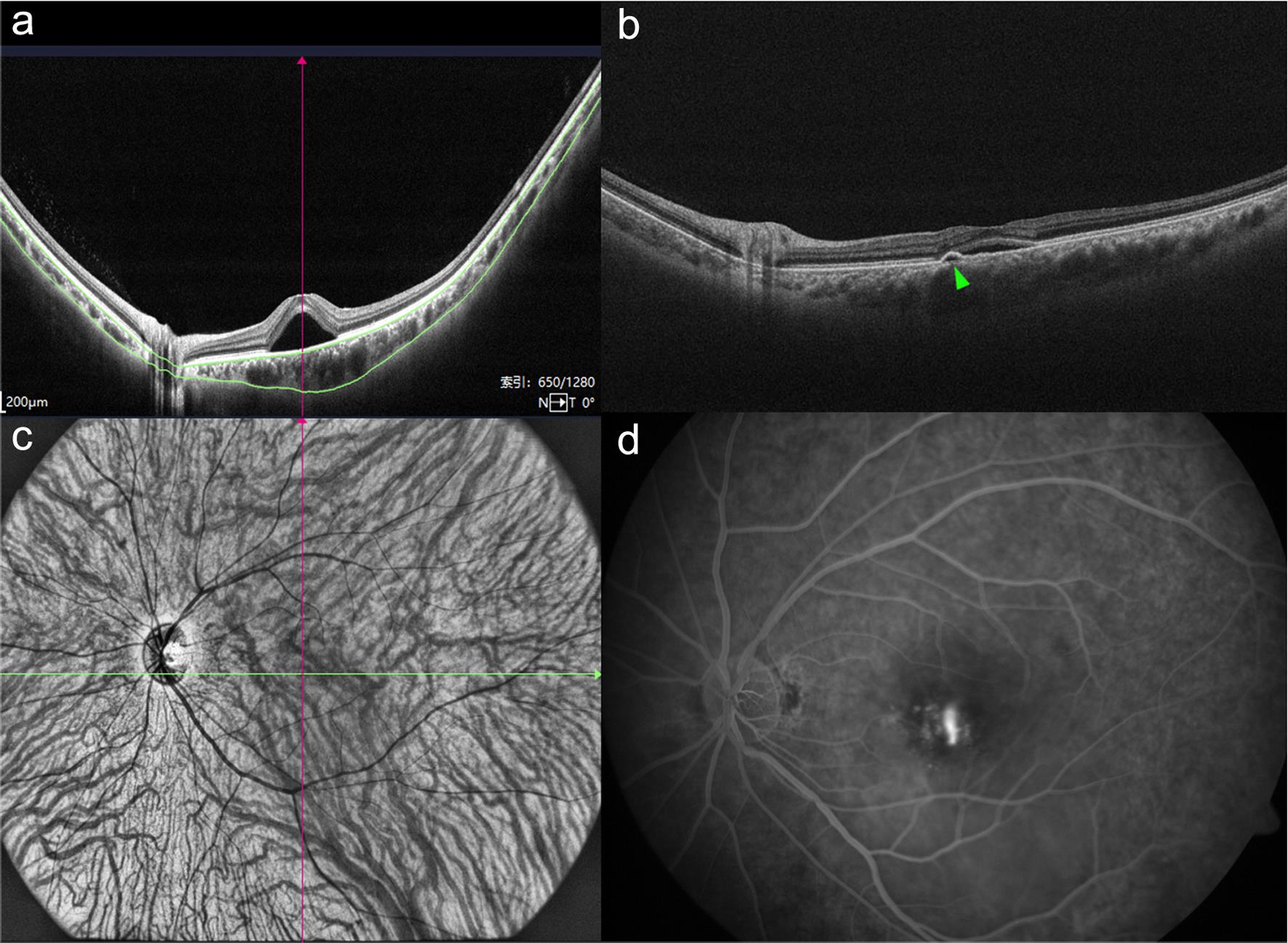
(a) An optical coherence tomography (OCT) image showing an increased subfoveal choroidal thickness. (b) An OCT image showing retinal pigment epithelial detachment (green triangle). (c) An ultrawide-field indocyanine green angiography image showing dilated choroidal vessels. (d) A fundus fluorescein angiography image showing leakage of dye appearing as a typical “smoke stack.”
FFA
Fluorescein angiography is an important diagnostic tool for CSC.3,6 Acute CSC typically presents as “smoke stack” or “ink blot” fluorescein leakage, meaning that the gradual leakage of dye can be observed with a “smoke stack” or “ink blot” pattern from the insufficiently connected RPE, with multifocal leakage being uncommon.5 In the mid-late stage of FFA, a strong fluorescein pool forms in the SRD area due to fluorescence accumulation.7 Chronic CSC is characterized by diffuse retinal pigment epithelium (DRPE) changes, in which the RPE shows atrophy, absence, and proliferative changes, and the affected area tends to expand over time. A study by Polak et al. suggests that a DRPE might be associated with the use of glucocorticoids.8 In addition, some scholars have determined the severity of CSC by the extent of DRPE, and a cumulative DRPE range exceeding five disc diameters in the macular area is considered severe chronic CSC.9 In the early stages of chronic CSC, FFA reveals an enhanced background fluorescence in the choriocapillaris of the lesion area, indicating that the underlying dilated, highly permeable choriocapillaris vessels can be observed due to RPE atrophy. At the corresponding site, an enhanced window defect can be seen. In the mid-late stage of chronic active CSC, FFA presents as unclear and slow-progressing fluorescein leakage within diffuse window defects and RPE staining, which can be multifocal in nature, with the fluorescence gradually increasing over time. In the late stage, FFA presents as patchy granular strong fluorescence. In areas of RPE proliferation and sub-RPE chronic fibrous exudation, FFA always presents as blocked weak fluorescence.
ICGA
ICGA is widely used in the diagnosis and treatment of CSC as well as the identification of differentiation with choroidal neovascularization (CNV) in CSC.10,11 A characteristic manifestation of CSC is that early in ICGA, dilated choroidal vessels show clear boundaries of strong fluorescence, corresponding to the areas of RPE atrophy or elevation on OCT. Meanwhile, in mid-term ICGA, due to the high permeability of dilated vessels, the boundaries of the strong fluorescence area become blurred, making the location of the dilated choroidal vessels unclear.12 In late ICGA, the area of strong fluorescence in the middle stage evolves into continuous strong fluorescence, washout-like changes, or eccentric migration of strong fluorescence, forming a ring of strong fluorescence. The strong fluorescence area in ICGA corresponds to the area of autofluorescence (AF) changes.13 It is also possible to observe weak fluorescence caused by a delay in the filling of choroidal arteries and capillaries,13 which continues into the middle and late stages of angiography. The region of RPE atrophy on ICGA is shown as weak fluorescence, which can first be differentiated at approximately 10 min and becomes more obvious in the later stages. This weak fluorescence is thought to be the result of hypoperfusion of choroidal capillaries.12 Compared to FFA, the typical “smoke stack” leakage characteristic of acute CSC appears later and has a smaller leakage area in ICGA.
In recent years, ultrawide-angle ICGA has been used to observe dilated choroidal vessels extending to one or more vortex vein ampullae before leaving the scleral margin, indicating possible obstruction of the vortex vein reflux.14 Some scholars have found that in diseases of the choroidal hypertrophy spectrum, the affected vortex veins dilate and leak, draining into the dilated ampullae, suggesting that the dilated choroidal vessels observed in ICGA are actually branches of the vortex veins; moreover, obstruction when the vortex veins pass through the sclera causes vortex vein stasis.15
Hayreh divided the vortex veins into four quadrants according to the horizontal and vertical watersheds, with each quadrant drained by 1–2 vortex veins.16 The horizontal watershed runs through the macula and the optic disc. In CSC, it is common to see asymmetrical dilation and anastomosis of the upper and lower vortex veins at the watershed; this anastomosis across the watershed is thought to be a common compensatory response to chronic vortex vein stasis, and vortex vein anastomosis is also considered a key factor in the development of diseases of the choroidal hypertrophy spectrum.15 The choroidal thickness in the macular area of chronic CSC is significantly thinner than that in acute CSC, suggesting that anastomosis at the watershed relieves vortex vein stasis through compensation, thinning the choroidal thickness.17 Anastomosis of the upper and lower vortex veins can be seen in 90% of thick choroid spectrum diseases, but the diameter of the vortex veins is the largest in CSC patients. These findings also suggest that as thick choroidal spectrum diseases progress, vortex vein stasis may gradually improve with anastomosis of the upper and lower vortex veins.17
Hiroe and Kishi found that all CSC-afflicted eyes have an asymmetry of the vortex veins, far greater than the 38% in normal eyes, and the high permeability area in ICGA corresponds to the dilated vortex veins, suggesting that asymmetric dilation of the vortex veins and reflux obstruction may increase the permeability of the choroidal capillaries in the macular area, thus acting as a triggering factor for CSC.18
OCT
OCT can noninvasively, efficiently, and repetitively present high-quality retinal images, intuitively reflecting the scope and degree of macular region SRD, making it a prime imaging method for the diagnosis and follow-up of active CSC.19 Spectral domain OCT (SD-OCT) provides high-definition images of pathological changes in the macular region’s retinal layers, and enhanced depth imaging mode renders all layers of the choroid observable, enhancing vascular morphology analysis and thickness measurement of the choroid.
The typical OCT presentation of acute CSC includes subretinal fluid (SRF) localization in the macular area, whereas a relatively long-term CSC often shows a subretinal strong reflective substance, thought to be fibrin deposits.20 A strong reflective substance continuous with the neuroretinal layer in the SRD area is considered an extension of photoreceptor outer segments, manifesting as subretinal yellow-white deposits at the fundus, corresponding to enhanced AF.21 After SRF absorption, the persistent elongation of the outer segments may progress into permanent subretinal deposits, leading to a poorer visual prognosis in patients.22 Strong reflective points can be observed between the subretinal cavity and the retinal layer of SRD.
In the evolution of acute CSC, strong reflective points in the retina can migrate from the inner layer to the outer layer and disappear concurrently with SRD; chronic and recurrent CSC are more likely to have strong inner retinal reflections. Chronic CSC presents some characteristic OCT features. Studies have found that roughly 58.8% of CSC patients have cystoid macular edema involving the foveal center, showing on OCT as macular retinal cystoid gaps without the corresponding fluorescein leakage.23 This characteristic presentation might be induced by local choroidoretinal adhesion, facilitating choroidal fluid leakage into the retina, and preventing area retinal detachment.24 Retinal cystoid degeneration can disappear or change, indicating fluid passing through the insufficiently compensated RPE as the cause. It has been found that CSC with a long course can lead to thinning of the outer nuclear layer outside the fovea, which affects visual function recovery after treatment.25
CSC patients are often accompanied by retinal pigment epithelial detachment (PED), with an incidence rate of 9–100%.26 At the FFA leakage point of active CSC patients, SD-OCT almost invariably shows minor RPE undulation or variably high PED. This RPE alteration is also seen in asymptomatic contralateral eyes.27 Due to differences in individual choroidal static pressure and the various stages of CSC, PED morphology is variable. Not only is smooth “dome-shaped” PED or small flat PED visible, but irregular wavy PED can be found as well.28 In some areas of the “dome-shaped” PED margin, minor RPE tears can be seen. During the acute phase, the RPE layer presents a uniform signal; while in the chronic stage, due to RPE hypertrophy, atrophy, and residual unabsorbed protein, the signal can increase, decrease, or be absent.29 In severe chronic CSC patients, diffuse RPE atrophy areas tend to expand with disease progression.9 Previous research has found that one-third of patients have multiple small PED areas outside of the SRD range, suggesting a wider spread of RPE functional disorder compared with the SRF range.30 The signals under the RPE protuberances vary significantly; weak reflective signals are often seen under the “dome-shaped” or flat PED of acute CSC, while medium reflective signals of irregular PED are mainly seen in chronic CSC. Irregular PED can present a “double-layer sign” on OCT, which has been reported to be present in 63% of acute CSC patients and 87% of chronic CSC patients.31 In the “double-layer sign” of chronic CSC, it is common to find neovascular components that can be diagnosed as hypertrophic CNV lesions. Unlike the polypoidal choroidal vasculopathy patients’ “double-layer sign,” which usually has strong interlaminar signals, the “double-layer sign” of CSC patients usually has medium-to-weak interlaminar signals.
Compared with healthy individuals, the choroids of both eyes of CSC patients are thicker. In individuals with unilateral affliction, the choroid of the affected eye is thicker than that of the opposite eye.32 Areas of choroidal thickening on OCT often show local or diffuse dilation of the outer choroidal large vessels. The corresponding inner choroid (including the middle vessel layer and capillary layer) is thinner.33 This may be due to the oppression of the outer layer’s large vessels causing atrophy (degeneration) of the inner layer vessels. The RPE in the area corresponding to the dilated outer layer large vessels is often elevated, indicating potential underlying mechanical pressure causing RPE compression. Although CSC is sometimes accompanied by an increase in choroid thickness, it is not a diagnostic criterion as typical acute CSC may not have an enlarged choroid. Factors like age, ocular axis, and refractive errors can all affect the choroidal thickness. In post-recovery CSC or CSC after treatment, the choroid thickness can often be seen to decrease.33,34
In chronic CSC, granular strong reflective signals are observed at the choroidal vascular wall using SD-OCT enhanced depth imaging, suggesting possible vascular wall structural changes. These strong reflective points often decrease or disappear with the absorption of SRF.26
In recent years, researchers have proposed the choroidal vascularity index, i.e., the ratio of the choroid lumen areas to the choroid cross-sectional areas to reflect the status of the choroidal vessels. This aids in the diagnosis and the observation of treatment effects of CSC. Studies have found a rise in the choroidal vascularity index in acute CSC patients as well as an increase in the contralateral eye choroidal vascularity index compared with age-matched healthy subjects.35
Research also has found a thickened sclera in CSC patients using anterior segment OCT, suggesting that a thick sclera may play a role in CSC development.36 Furthermore, there are recent reports of ciliary body choroidal leakage and the subsequent accumulation of fluid in the supra ciliary body choroidal space in CSC patients.37
En face OCT
In recent years, several scientists have applied en face OCT to observe CSC.38 The SRF and PED in CSC are presented in en face OCT as areas of weak signal with clear boundaries. In some SRFs, concentric circles of strong reflection and point-like strong reflection signals can be observed, which may be found on the outer segments of photoreceptor cells or on the RPE surface.39 En face OCT can show weakly signaled tube-like structures corresponding to the middle and large vessels in the Sattler and Haller layers under the RPE in SD-OCT. In comparison to acute CSC, chronic CSC exhibits more diffuse choroid outer layer blood vessel expansion (18.2% vs. 66.6%). In the areas of choroid signal attenuation in SD-OCT, en face OCT can reveal choroid vessel dilation in the inner layers as well.40
In the images of en face OCT, the choroid outer layer vessels correspond to vortex veins. By comparing features of wide-field en face OCT and ultrawide-field ICGA images in CSC patients, Ramtohul et al. found that en face OCT could accurately display the vortex vein morphology and quantitatively measure the vortex vein drainage areas.38 These corresponded to the Haller layer, large vessel hyperpermeable areas, and areas of delayed choroidal filling in ICGA. On the choroidal thickness map, en face OCT also revealed blood stasis, asymmetric dilation, and watershed area anastomosis in the entire vortex vein drainage area, which on ICGA corresponded to dilation and increased permeability.38 The findings from en face OCT consistent with the theory of chronic vortex vein stasis in pachychoroid spectrum disease, proposed recently based on ICGA findings, suggest that en face OCT can serve as a noninvasive alternative to ICGA examination to some extent.
Although the images from en face OCT are synthesized, with C-scanning and three-dimensional imaging technology, more stereoscopic information about the various levels of retinal and choroidal structures can be obtained. Hence, it remains crucial to observe CSC retinal and choroidal lesions more intuitively and noninvasively.
OCTA
OCTA plays a significant role in the choriocapillary blood vessel and blood flow monitoring in CSC and serves a crucial function in the differential diagnosis of chronic CSC.41 For acute CSC, OCTA abnormalities in the blood flow occur at the level of the choriocapillary. Below the SRD, an increase in the choroidal blood flow may indicate an active disease area. Numerous OCTA studies have found an irregular blood flow in the choriocapillaris layer of CSC patients, which is characterized by regions of weak blood flow signal wrapped in strong blood flow signal areas.42 The region of strong blood flow signal in the choriocapillaris layer corresponds primarily with the hyperperfused zone in ICGA and is marked by vascular dilation and increased blood flow; regions of weak blood flow signal frequently appear as shadows with a decreased flow density, typically matching the areas of retinal or RPE detachment.43
In contrast, for chronic CSC, OCTA may reveal local filling defects at the level of the choriocapillaris, accompanied by dilated arterioles and venules, which may persist even after the regression of the SRD.42 For chronic CSC, the diagnosis of concurrent CNV is challenging. The presence of SRF and irregular PED accompanied by irregular hyperfluorescence during the angiographic process in FFA and ICGA may confound the judgment of CNV and interfere with differential diagnosis. However, OCTA, through its layer-by-layer imaging, can enhance the sensitivity of CNV detection. Swept-source OCTA is currently considered the optimal choice for detecting CNV secondary to chronic CSC.44 In a study including 29 chronic CSC patients, the proportion of secondary CNV reached as high as 34.5%.45
In CSC, OCTA reveals dark areas and dark spots at the level of the choriocapillaris, corresponding to SRD and nonexudative PED, subretinal deposition, choroidal concavity, choroidal effusion, choroidal atrophy, etc.46 These anomalies do not display a detectable signal flow, enabling differentiation from CNV. In the OCT examination of chronic CSC, a “double-layer sign” is often seen. Layer-by-layer analysis by OCTA can differentiate whether there are vascular components between its layers, differentiating it from polypoidal choroidal vasculopathy, etc. Notably, whether the abnormal vessels detected by OCTA in the choriocapillaris layer of chronic CSC patients after increased activity are actual neovascularizations or images produced by choroidal vessel dilation postexercise is still unknown.
Of course, OCTA comes with its limitations. The appearance of SRD may cause segmentation errors, and choroidal images may require manual segmentation, increasing the challenges of use and analysis.
In photodynamic therapy for CSC based on imaging examination, OCTA can also play an important role as a noninvasive alternative. Historically, photodynamic therapy for CSC typically relied on the hyperpermeable vascular areas identified by ICGA to guide treatment. Recent research, however, suggests that the coarse granular highly reflective area in the choriocapillary layer visible in en face OCTA aligns well with the hyperpermeable areas on ICGA, offering a viable alternative to ICGA.47
FAF
RPE lipofuscin generates short-wavelength AF; thus, FAF imaging can reflect the metabolic function of the RPE.48 In acute CSC, approximately 70–100% of involved eyes exhibit localized hypo-AF at the point of leakage, suggesting the possibility of localized RPE damage or slight tearing in these regions.14 In chronic CSC patients, the lesion site often shows granular hypo-AF and mixed dot-like AF. In chronic active CSC, regions of hyper-AF matched with SRD regions can be seen, indicating the presence of prolonged RPE injury.
In chronic CSC, FAF can show several elliptical hypo-AF lesions, presenting a gravity tract aspect with hyper-AF usually surrounding it. This may be related to residual chronic SRF movement and light receptor cell damage in the hyper-AF area, but without RPE damage.49 These changes in AF that present variations in intensity (alternatively strong and weak) similar to gravity paths often originate from the macula and the optic disc.14 After CSC self-recovery, granular residual hyper-AF can still be seen, usually matching the strong reflecting points in the inner retinal layer as observed on SD-OCT. This is likely related to photoreceptor cell outer segment loss.
Ultrawide-field FAF can better display the involvement of the peripheral retina, with research indicating that it occurs in more than 50% of patients.49 In cases of CSC with only one affected eye, changes in AF due to overlooked peripheral retina involvement or localized RPE changes can still be seen in the opposite, unaffected eye.
Near-infrared AF, another noninvasive imaging examination technique based on choroidal and RPE pigment generating AF, uses a diode laser with a wavelength of 788 nm for excitation and a shielding filter for detecting emission light greater than 810 nm. Near-infrared AF differs from short-wave AF in terms of fluorescence clusters. It mainly reflects the image of melanin distribution and also reflects the RPE status.50 Although not frequently performed on CSC patients, near-infrared AF can display an initial AF reduction in acute CSC patients, suggesting that long-term changes may occur in the choroidal pigment following CSC.51,52
Recently, fluorescence lifetime imaging technology has been used for fundus examinations. This technique stimulates the retina’s AF through pulsed lasers, where the fluorescence lifetime detected represents the duration of the excited fluorescence cluster at its higher energy level. Based on this technology, a fluorescence lifetime imaging ophthalmoscope (FLIO) can produce images of endogenous retinal fluorescence cluster lifetimes. Researchers have used this technique to observe CSC at various stages by measuring the lifetime of the retinal fluorescence clusters, which allows them to monitor retinal changes caused by CSC. During the very early stage of acute CSC, the fluorescence lifetime imaging ophthalmoscopy results are similar to those of normal controls. However, as the duration of the disease progresses, the fluorescence lifetime in the SRD area decreases, while the retinal changes in chronic CSC may result in an extended fluorescence lifetime.53 Fluorescence lifetime imaging can be used as a noninvasive diagnostic tool to monitor the changes caused by CSC within the retina, although its use is not widespread in current clinical practice.
Multispectral fundus imaging
Multispectral fundus imaging utilizes an array of monochromatic light-emitting diode light sources to project onto different depths, including the RPE layer and choroid, of the retinal tissue.54 The different tissues absorb varying spectrums of light. Monochromatic light reflection images from different depths of the retina are collected to form monochromatic spectral images, presented in an en face format. In the case of CSC, different wavelengths of light can be used to observe the different levels of the retinal and choroidal structure. The features of multispectral fundus imaging are as follows: the yellow-green spectrum can display the range of neuroretinal detachment and the yellow-white exudate within the detachment cavity; and the 550–580 nm spectrum can clearly show the detachment of the neuroepithelial layer and the exudation within the detachment cavity. The abnormal or microtear areas of the RPE appear as dark spots, which align with the fluorescent leakage point of FFA. Red and infrared spectra can observe the RPE and choroid vascular structure. Wavelengths of 620 nm and above can observe dilated choroid blood vessels, which are clearer at wavelengths less than 810 nm, showing good consistency with what can be observed in ICGA.55 While some researchers suggest that multispectral fundus imaging can fully replace FFA in guiding clinical diagnosis and treatment, it should be noted that artifacts and various optical media changes may cause interference.55
Conclusion
Fundus imaging examination techniques play a significant role in the diagnosis, classification, differential diagnosis, treatment guidance, and follow-up observation of CSC patients. Multi-modal imaging enables the effective integration of the main retinal and choroidal imaging technologies, thereby deepening our understanding of CSC and its pathogenic mechanisms. In recent years, advances in OCT technology have enhanced the depth, breadth, and precision of scanning and have facilitated vascular imaging, thus providing a valid supplement and partially noninvasive alternative to FFA and ICGA examinations. By summarizing the characteristic features of CSC multi-modal imaging, the scientific and reasonable application of multi-modal imaging in CSC is facilitated.
With technological advancements, continuous improvements and innovations in examination techniques will emerge, and multi-modal imaging examination and analysis for CSC will become more precise and detailed. Combined with clinical feature observation and genetic analysis, these advances will provide a more reliable basis for the standardized diagnosis and personalized treatment of CSC patients.
Abbreviations
- AF:
autofluorescence
- CNV:
choroidal neovascularization
- CSC:
central serous chorioretinopathy
- DRPE:
diffuse retinal pigment epithelium
- FAF:
fundus autofluorescence
- FFA:
fundus fluorescein angiography
- ICGA:
indocyanine green angiography
- OCT:
optical coherence tomography
- OCTA:
optical coherence tomography angiography
- PED:
pigment epithelial detachment
- RPE:
retinal pigment epithelium
- SD-OCT:
spectral domain optical coherence tomography
- SRF:
subretinal fluid
Declarations
Acknowledgement
None.
Funding
None.
Conflict of interest
The authors have no conflicts of interest related to this publication.

 Author information
Author information