Introduction
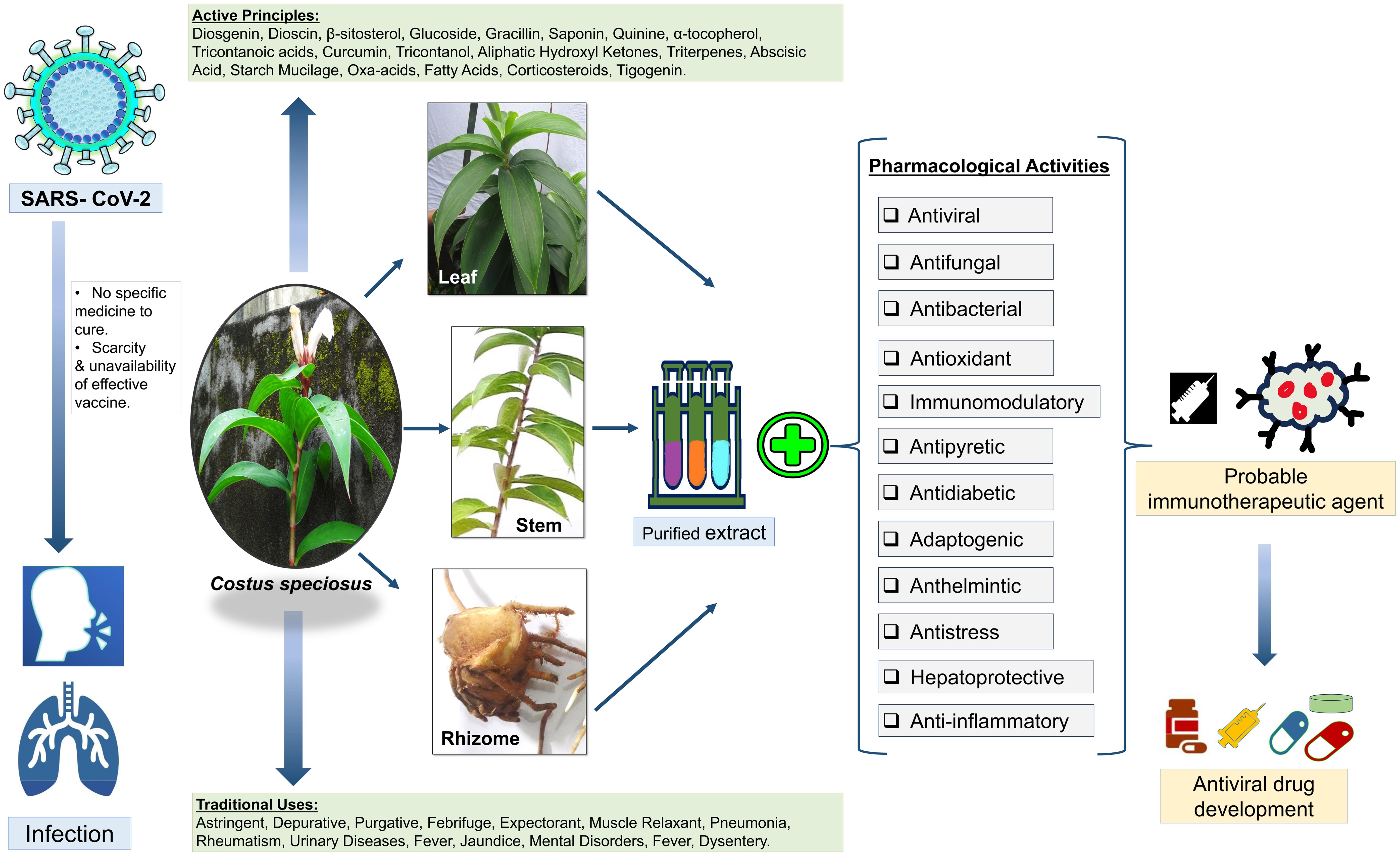
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a single-stranded positive-sense RNA virus that is very contagious and has quickly spread globally; this disease results in severe mortality and has no specific cure.1 The effective management of COVID-19 is still not in control and is not feasible with the recurrent mutations of SARS-CoV-2, even after applying continuous vaccination drives and treatment with drugs that are available in the market.2 To discover a potential and appropriate drug for COVID-19, scientists have been working hard; however, to date, no effective methods for the prevention and therapeutic management of COVID-19 infection have been reported. Owing to the scarcity and unavailability of effective vaccines, antiviral drugs, effective prophylactic therapies, and remedies are still required to prevent SARS-CoV-2 infection; thus, the burden of the COVID-19 pandemic shined a light on the need to develop alternative preventive and treatment options.
Nowadays, the World Health Organization focuses on developing effective treatments and welcomes innovative therapies giving more emphasis to traditional medicines as traditional medicinal plants are the largest reservoir of biologically active secondary metabolites, which play an important role in curing different diseases including diseases caused by viruses from ancient times.3 Ayurveda, Unani, and Siddha are the most ancient indigenous systems of medicine of human civilization used to treat various disorders for many centuries using a variety of medicinal plants with their preventive, curative, and rehabilitative properties.
Costus, from the family Costaceae and order Zingiberales, is a large genus comprising over ∼150 species that has a long ethno-medicinal account in India and has been used in traditional systems of medicine—Ayurveda, Unani, and Siddha—since ancient times. According to the Ayurvedic Pharmacopoeia of India (2008),4Costus is referred to as “kebuka” and has properties like rasa, guna, virya, vipaka, and karma; it is used in formulations like krmighna, kvatha, and curna against agnimandya, slipada, arsa, grahani, jvara, kamala, kasa, kustha, raktavikara, krimiroga, and kaphaja, among others.
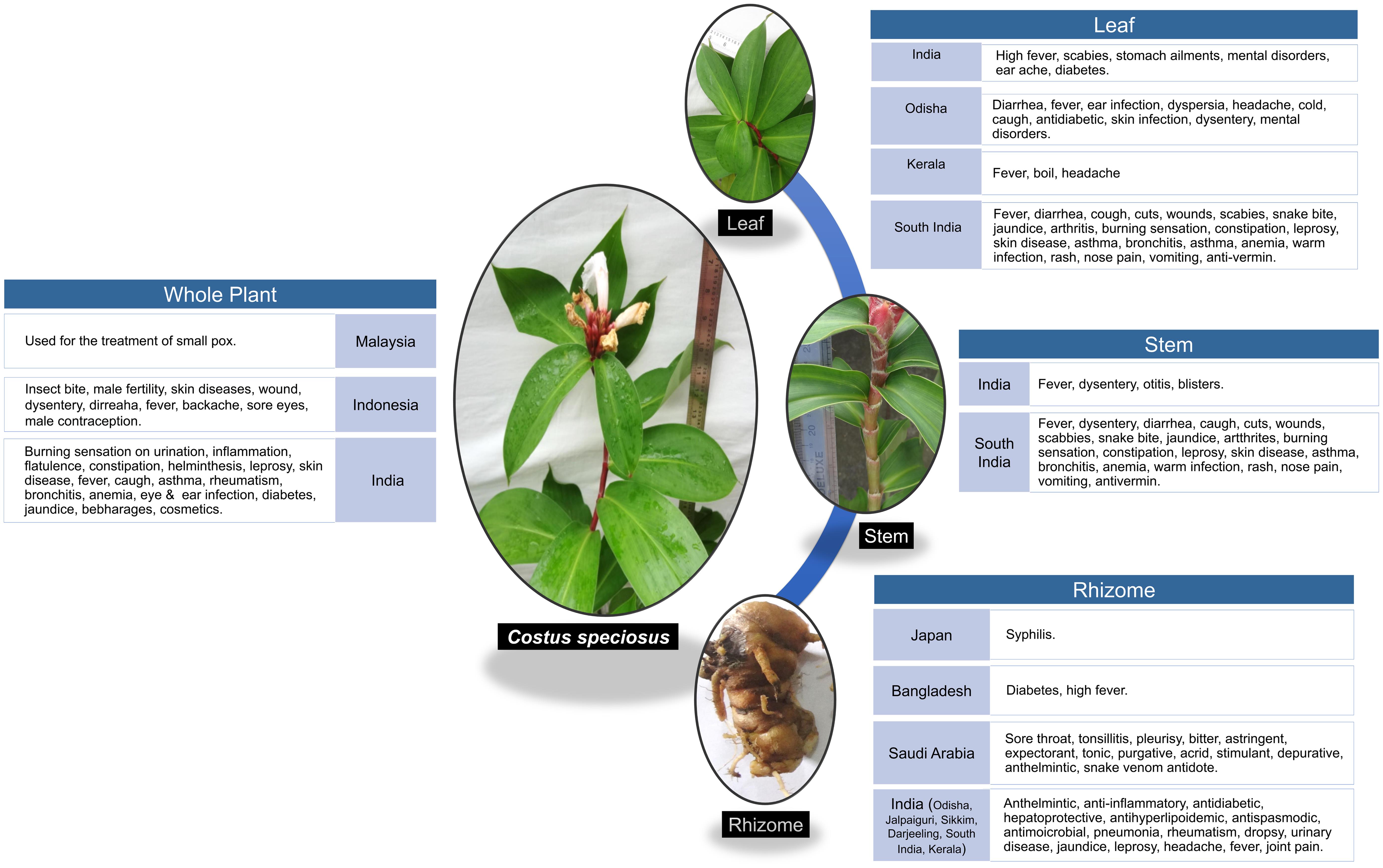
Costus speciosus (C. speciosus) Koen. ex Retz. is a perennial herb with a tuberous horizontal rhizome and spirally arranged simple leaves with bisexual flowers.5 It exhibits multiple cytotypes, occurs worldwide,6 and grows wild in shady places of mixed deciduous forests in India.7 Rhizomes contain high amounts of diosgenin.8 The active ingredients include diosgenin, dioscin, β-sitosterol, glucoside, gracillin, saponin, quinine, α-tocopherol, tricontanoic acids, curcumin, tricontanol, aliphatic hydroxyl ketones, triterpenes, abscisic acid, starch mucilage, oxa-acids, fatty acids, corticosteroids, and tigogenin. Traditionally, the plant has been used as an astringent, depurative, purgative, febrifuge, expectorant, and muscle relaxant. In addition, it has been used for the treatment of pneumonia, rheumatism, urinary diseases, fever, jaundice, mental disorders, fever, and dysentery (Fig. 1).9 The phytochemicals presented in C. speciosus possess a number of activities, such as antiviral, antibacterial, antifungal, antioxidant, immunomodulatory activity, antipyretic, antidiabetic, adaptogenic, anthelmintic, antistress, hepatoprotective, anti-inflammatory, and many other biological activities,9 demonstrating its potential for the prevention and therapeutic usefulness in the treatment of COVID-19.
In this current scenario, as there is no registered medicine to treat COVID-19,1C. speciosus could be a promising antiviral component to prevent and cure SARS-CoV-2 infection without any harmful side effects. This review compiles all of the information regarding the potential of C. speciosus as an effective therapeutic agent to fulfill the global demand to prevent or treat COVID-19 and can serve as a guide for further research for the early development of new drugs and clinical applications to combat this deadly disease.
Distribution
The plant C. speciosus is widely distributed in the humid tropical and subtropical regions on both sides of the equatorial plane.5,10 It is exclusively distributed throughout the globe, especially in the tropical regions of Asia, Africa, and the Americas.11,12C. speciosus is region-specific and native to the Malay Peninsula of Southeast Asia,13 India, Indonesia, Sri Lanka, and Malaysia; however, it has been naturalized in some other tropical areas, including Hawaii.11 Within India, it grows from the central and eastern Himalayas to southern India and is also located in the Andaman and Nicobar Islands.14C. speciosus is generally distributed below an altitude of 1,500 m in tropical forests throughout the Indian subcontinent,15 mostly in moist, warm, and hot evergreen forests. The geospatial distribution in the country ranges throughout to the foothills of the Himalayas from Himachal Pradesh to Assam; the Vindhya Satpura Hills in central India; the Eastern Ghats of Andra Pradesh; the Western Ghats of Maharashtra, Karnataka, Tamil Nadu, Kerala, Meghalaya, Bihar, and Khasi; as well as the Jaintia Hills, Uttaranchal, Orissa, MP, North Bengal, West Bengal, and Himalayan tracts,11 excluding the arid and semiarid areas of Punjab, Haryana, Rajasthan, Gujarat, and the Peninsular India (Table 1).9–28 This plant thrives well on rich moist soil in shady localities under deciduous forests; hence, it is located wildly in the soggy wet-lands, particularly in the streams and river banks.16 It grows well in a climate with high humidity16 and is found in road-side ditches and low-lying areas10 in the marshy and shady places17 of forest plantation. In many regions, this plant is also cultivated for its ethno-medicinal and ornamental purposes.9,11,16
Mode of uses in different parts of Costus speciosus in various regions of the world
| Location | Parts used | Mode of use | References |
|---|---|---|---|
| Japan | Rhizome | Rhizome extract is used for controlling syphilis. | 13,22 |
| Malaysia | Whole plant | The plant is used for small pox. | 9,15 |
| Indonesia | Whole plant | This plant is commonly used to treat insect bite, to inhibit male fertility, skin diseases, wounds, dysentry, diarrhoea, fever, backache, sore eyes and also used as a traditional male contraception in some areas. | 16 |
| Bangladesh | Rhizome | The rhizome is used for diabetes and high fever. | 25 |
| Saudi Arabia | Rhizome | In Saudi folk medicine, the rhizome is used for the treatment of sore throat, tonsillitis, and pleurisy. This plant is also traditionally used as a bitter, astringent, expectorant, tonic, purgative, acrid, stimulant, depurative, anthelmintic, and expectorant. C. speciosus is one of the most effective traditional medicinal plants in Islamic medicine. Costus has been mentioned in the Prophet’s medicine for treating pharyngitis and tonsillitis in children, pleurisy, and as an antidote for snake venom. | 12,23 |
| India | |||
| Leaf; Stem; Root; Rhizome | C. speciosus is one of the constituents of the indigenous drug “amber mezhugu,” which is useful in treating rheumatism. Bruised leaves are used for bathing or topically applied to reduce fever in patients with high fevers. The leaves are also used to treat scabies, stomach ailments, mental disorders, and earaches. Diabetic people in India eat one leaf daily to keep their blood glucose levels low. The decoction of the stem is used to control fever and dysentery. The juice of the freshly burned stem is used as an ear drop to heal otitis. The stems are ground into a paste and applied to blisters. In Indian traditional medicine, the rhizomes and roots of C. speciosus are believed to have anthelmintic, anti-inflammatory, antidiabetic, hepatoprotective, antihyperlipidemic, antispasmodic, and antimicrobial activities. Rhizomes are given in pneumonia, rheumatism, dropsy, urinary diseases, jaundice, and diabetes. The juice of the rhizome is traditionally given with sugar to treat leprosy, worm infestations, abortion, and applied to the head for cooling and relief from headaches. The decoction of the rhizome is used for crushing kidney and bladder stones. The plant is used in traditional medicine to treat burning sensation during urination, inflammation, flatulence, constipation, helminthiasis, leprosy, skin diseases, fever, persistent cough, asthma, rheumatism, bronchitis, inflammation, and anemia. Internally, the plant is used for eye and ear infections, diarrhea (sap from leaves, young stems), colds, catarrhal fever, cough, dyspepsia, and treating boils. It is also used as an ingredient in cosmetics to enhance sexual attractiveness, as mentioned in KamaSutra. In India, herbal preparations of C. speciosus are currently used by local people in tea bags (herbal-chai), supplements, beverages, and food items that regulate body sugar and cholesterol levels. It is also used locally for diabetes and jaundice. | 9,12–15,17,20 | |
| Odisha | Rhizome; Leaf | The rural and tribal people of Odisha use the rhizome of C. speciosus as a raw vegetable or as a cooked vegetable, and it is highly nutritious with a high content of carbohydrates, starch, amylase, protein, and lipids/oil. The tribal communities of the state used the rhizome to cure joint pain, skin infections, and consumed it as a nutraceutical. Rhizomes are also given in diseases such as pneumonia, expectorant, for curing asthma, fever, bronchitis, leprosy, skin diseases, rheumatism, dropsy, skin diseases, snake bites, urinary diseases, jaundice, among others. The rural and tribal people use the leaves against diarrhea, fever, ear infections, dyspepsia, headache, cold, and cough. The leaves are reported to possess antidiabetic properties and are used against skin infections, dysentery, and to aid in mental disorders, etc. It is not only used as therapeutic medicine but for other socio-cultural purposes, such as wrapping indigenous food items, mat making, and treatment of evil repellents. | 18 |
| West Bengal | Rhizome; Stem | Rhizome is used to treat red urine, dyspepsia, and diabetes. The peeled stem is chewed to relieve thirst in the jungle. | 11,24,26 |
| Darjeeling | Rhizome | The rhizome decoction is used by the tribes of Darjeeling. | 11,27 |
| Kerala | Leaf; Rhizome | The fresh juice of the plant leaf and rhizome is used in Kerala. | 28 |
| Eastern Himalayan | Whole plant | C. speciosus is one such plant used by the locals of the regions of eastern Himalayan belt. | 19 |
| Sikkim hills | Rhizome | Rhizome decoction is used by the tribes of Sikkim hills. | 27 |
| Eastern Hima layan | Whole plant | C. speciosus is one such plant used by the locals of the regions of eastern Himalayan belt. | 19 |
| South India | Stem; Leaves; Rhizome | This plant is used as food and medicine by the Kannikars, the primitive hill tribes of South India. The juice of the rhizome is applied to the head for cooling and relief from headaches. A decoction of the stem is used for fever and dysentery. Bruised leaves are applied during a fever. Patients with high fever mostly use leaf infusion or decoction as a sudorific or in a bath. The sap from leaves and young stems is used against diarrhea, coughs, cuts, wounds, scabies, snake bites, jaundice, arthritis, burning sensations, constipation, leprosy, skin diseases, asthma, bronchitis, inflammations, anemia, worm infections, rashes, nose pain, to stop vomiting, and as an anti-vermin and for abortion. | 10,21 |
Morphology of C. speciosus
C. speciosus Smith belongs to the family Costaceae and is an ornamental, perennial, rhizomatous, erect, succulent, herbaceous, and monocotyledonous plant; it grows up to 2.7 m in height, arising from a horizontal rhizome.9 Tuberous rhizomes clothed with sheaths are found in the lower parts, whereas the upwards parts are leafy.29 The upper surface of the rhizome is marked with circular nodal scars with remnants of leaf bases; on the other hand, the lower and lateral surfaces exhibit small circular scars of roots or a few wiry fractured yellowish-brown rootlets.30 The stem is subwoody at the base.14 The leaves are simple, dark green in color, elliptical to oblong or oblong to lanceolate, thick, subsessile, and silky pubescent beneath, and spirally arranged on the stems; the leaf sheaths are coriaceous. The flowers are bisexual, large, and white, and they occur in cone-like terminal dense spikes, with bright red bracts and a lip with a yellowish throat.31 The flowers look like crape paper; thus, the common name of the plant is “Crape ginger.” Flowering generally occurs during the months of July to October.32 The attractive red cone-shaped bracts remain attached to the inflorescence, even after the flowers fade away. The fruits are capsular, globosely trigonous, and red in color. The seeds are black, with white aril. The style is filiform, the stigma generally has a semilunar ciliated depression, and the ovary is generally three-celled. The ovules are many and superposed.5,9,18,33 A detailed overview of all of the probable mechanistic details of C. speciosus as well as salient findings is also provided in Table 2.11,12,15,17,19–23,34–50
The detailed overview of all the probable mechanistic details of Costis speciosus with their salient findings
| Sl No. | Plant related activity | Reference | Duration of the study | Years of literature reviews | Salient findings | Inclusion & exclusion criteria |
|---|---|---|---|---|---|---|
| 1. | Anti-inflammatory activity | 12 | 2013–2014 | 1973 to 2017 | The methanolic extract of C. speciosus demonstrated potent anti-inflammatory effects in in vitro studies by inhibiting pro-inflammatory mediators in murine BV-2 cells. The aqueous solution of C. speciosus is also used as nasal drops to improve acute symptoms of pharyngitis and tonsillitis, highlighting its potential as a promising strategy for various inflammatory disorders. | Patients with acute pharyngitis and tonsillitis were included. |
| 34 | – | 1956 to 2014 | Ethanol leaf extracts of C. speciosus revealed the presence of alkaloids, tannins, saponins, steroids, terpenoids, flavonoids, and total phenols, indicating potential for anti-inflammatory activities. | – | ||
| 35 | – | 1955 to 1997 | Ethanolic extract of C. speciosus demonstrated significant anti-inflammatory effects, particularly in carrageenan-induced edema and cotton pellet-induced granuloma formation. | – | ||
| 36 | – | 1962 to 2011 | The anti-inflammatory activity of methanol extracts from C. speciosus aerial parts was evaluated using the carrageenan-induced paw edema test in rats. | – | ||
| 19 | – | 1974 to 2014 | The heat-induced haemolytic assay was conducted by incubating methanol extract of C. speciosus leaves at various concentrations with the RBC suspension. The percentage of haemolysis and membrane stabilization were calculated, indicating the concentration-dependent anti-inflammatory activity of the extract. | – | ||
| 37 | 1932 to 2013 | The denaturation of proteins, a known cause of inflammation, was assessed by subjecting the leaf extracts of C. speciosus in different concentrations and evaluating their ability to protect against protein denaturation and membrane stabilizing activity was determined by assessing the extracts’ impact on erythrocyte membrane stability. | – | |||
| 38 | – | 1968 to 2014 | The anti-inflammatory activity was assessed through in vitro studies such as membrane stability testing, hypotonicity-impelled HRBC hemolysis, protein denaturation inhibition and in vivo tests such as formalin-induced writhing test and tail immersion test using methanol extract of C. speciosus on selected healthy male rats. | – | ||
| 2. | Adaptogenic activity | 39 | Albino rats were treated for 16 days. | 1968 to 2008 | The study investigated the antistress activity of ethanolic extracts from C. speciosus rhizomes on albino rats subjected to cold immobilization stress using Soxhlet extraction. The extracts prevented neurotransmitter depletion, particularly norepinephrine and dopamine, and modulated MAO activity suggesting adaptogenic potential of the plant. | – |
| 12 | – | 1973 to 2017 | The study suggested that extracts from C. speciosus reduce stress-induced elevations of 5-HT and 5-HIAA levels, indicating a potential antidepressant effect. | – | ||
| 40 | 1966 to 1983 | The study investigated the impact of immobilization stress on the release of 5-hydroxyindoles (specifically 5-HT or serotonin) in the rat hippocampus using in vivo voltammetry. | – | |||
| 3. | Anti-diabetic activity | 11 | – | 1972 to 2016 | Ethanolic extracts of C. speciosus rhizome have shown effectiveness in reducing blood glucose levels and improving lipid profiles in rats. | – |
| 37 | – | 1932 to 2013 | The anti-diabetic activity assessment involved extracting compounds from C. speciosus leaves using ethanol. The compounds were identified through silica column chromatography and spectroscopic analysis. The study concluded by highlighting the potential of the identified compounds in treating diabetes. | – | ||
| 20 | – | 1956 to 2012 | The study focused on quantifying diosgenin, a steroidal saponin and major bioactive compound in C. speciosus using HPLC analysis. Diosgenin extraction involved hydrolysis and hexane extraction. HPLC was conducted on leaves and rhizomes, revealing variation in diosgenin content among species and plant parts. | – | ||
| 41 | The experimental period lasted for 30 days. | 1951 to 2008 | C. speciosus roots were extracted with hexane to isolate costunolide, which was administered to streptozotocin-induced diabetic rats. Costunolide demonstrated significant normo-glycemic and hypolipidemic effects, suggesting its potential as a drug for managing diabetes. | Male Wistar rats weighing about 190–200 g were included. Diabetic rats were included based on a fasting plasma glucose range. Rats outside the specified weight range and those not meeting the criteria for STZ-induced diabetes were excluded. | ||
| 21 | The experimental period three weeks. The rats were treated twice daily with the extracts. | 1974 to 2016 | Petroleum ether extract, ethanol extract and water extract of C. speciosus leaves were orally administered to streptozotocin-induced diabetic rats. Ethanolic extract exhibited significant antidiabetic effects in streptozotocin-induced diabetic rats. | Inclusion criteria involved the selection of streptozotocin-induced diabetic rats. | ||
| 42 | The experimental period lasted for 15 days, during which the rats were treated daily. | 1969 to 2008 | The ethanolic extract of C. speciosus rhizome was investigated using Soxhlet apparatus and demonstrated significant antidiabetic effects in alloxan-induced diabetic rats. | Inclusion: Wistar Albino rats weighing 150–180 g. Exclusion: Rats not developing hyperglycemia 48 hours after alloxan injection were excluded and replaced with new animals. | ||
| 22 | – | 1970 to 2009 | Aqueous, methanolic and hexane extracts of C. speciosus demonstrated efficacy in reducing serum glucose and improving other biochemical parameters in diabetic rats. Additionally, the leaves exhibit hypoglycemic properties and enhance insulin action. | – | ||
| 43 | – | 1954 to 2016 | Diosgenin quantification through HPTLC, and in vitro assessments including starch–iodine assay for antidiabetic activity, were performed with methanolic extract of C. speciosus. | – | ||
| 4. | Antimicrobial Activity | 34 | – | 1956 to 2014 | The ethanolic leaf extract revealed the presence of potential bioactivities, validating its traditional medicinal use and highlighting its potential in drug discovery. | – |
| 44 | – | 1972 to 2018 | Ethanolic extracts of C. speciosus rhizomes, particularly in high concentrations and obtained through both hot and cold methods, demonstrated significant antibacterial and antifungal activities against diverse pathogenic microorganisms. Aqueous extract on hot displayed antibacterial activity, with no antifungal activity. Aqueous extract on cold did not exhibit antibacterial effects against any tested microorganisms. | – | ||
| 45 | – | 1987 to 2007 | The study focused on the phytochemical screening, HPTLC separation, and antimicrobial screening of C. speciosus. Methanolic and aqueous extracts exhibited antibacterial activity against Staphylococcus aureus, with the traditional use of boiling in water supporting this finding. However, methanolic extract showed no inhibitory activity against other bacteria. | – | ||
| 46 | – | 1980 to 2012 | The study investigated the antimicrobial properties of C. speciosus roots, employing steam distillation for essential oil extraction, cold percolation for plant extracts, and epoxidation of diosgenin. Results demonstrated significant inhibitory effects on fungi and bacteria. | – | ||
| 47 | – | 1966 to 2011 | The essential oil extracted from the rhizome of C. speciosus was analyzed using GC-FID and GC-MS. The antibacterial screening demonstrated significant activity against both gram-positive and gram-negative bacteria. | – | ||
| 17 | – | 1973 to 2019 | The findings suggested the potential of antimicrobial activity was the leaves of C. speciosus. The agar well diffusion assay revealed significant antimicrobial effects, with the highest activity observed against E. coli. | – | ||
| 5. | Anthelmintic Activity | 48 | – | 1970 to 2010 | The article was a review that consolidates information on the phytochemical and pharmacological properties of C. speciosus. The methodology involves the study and investigation of available literature related to the plant. | – |
| 15 | – | 1970 to 2015 | Pheretima posthuman were employed as experimental worms. The anthelmintic activity of C. speciosus was assessed using methanolic and aqueous extracts of the aerial parts. The anthelmintic activity of the extracts was compared with a standard drug, albendazole (20 mg/ml). | – | ||
| 6. | Estrogenic Activity | 23 | – | 1972 to 2020 | The methanolic extract of C. speciosus rhizome used to examine the uterine weight of adult female rats. | – |
| 49 | – | 1940 to 2010 | The study presented data suggesting a significant stimulation of uterine activity by C. speciosus rhizome extract, with a focus on the role of its constituent, b-sitosterol, acting via a non-estrogen receptor-mediated mechanism. | – | ||
| 50 | – | 1970 to 2004 | The study provided information about the chemical composition of C. speciosus by different experimental methods such as-Liebermann-Burchardt test, HPLC, UV spectrum analysis, IR spectrum analysis, thin layer chromatography. | – | ||
| 7. | Antipyretic Activity | 35 | Drug administration occurred 10 hours after yeast administration. | 1955 to 1994 | Antipyretic properties were studied in yeast-induced pyrexia in rats. Only male albino rats were used for the antipyretic study, and rectal temperature was recorded. The ethanolic extract of the C. speciosus rhizome demonstrated significant anti-pyretic properties. | – |
Various pharmacological activities of C. speciosus
Anti-inflammatory activity
The term inflammation is classically defined as a complex biological response in which areas become reddened, hot, swollen, and often painful, especially as a reaction to injury or infection per se.51 It is well known that SARS-CoV-2 replication triggers the inflammatory response in the host cellular milleu.52 Acute lung injury during SARS-CoV-2 infection is due to aggressive inflammation initiated by viral replication and allied factors.53 When inflammation is overwhelming, it may lead to serious unfavorable outcomes or even death, as seen during the COVID-19 pandemic.54 Inflammation also plays a key role in the development and severity of COVID-19 when it occurs with other diseases (i.e., comorbidity).55 Thus, controlling or preventing the inflammatory response may be an effective way of preventing the life-threatening condition in patients with COVID-19. One of the keys to successfully manage the disease is to effectively control the inflammation rapidly with the appropriate anti-inflammatory drugs.56
Clinical evidence has shown that during COVID, uncontrolled inflammation turns into hyperinflammation and becomes chronic, which inhibits the adaptive immune responses and ultimately causes multiple organ dysfunction. Such dysregulated inflammation results in a “cytokine storm” that is manifested due to the release of high levels of pro-inflammatory cytokines, such as interleukin (IL) 1β, IL6, granulocyte colony-stimulating factor, interferon gamma-induced protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein-1 alpha, tumor necrosis factor-alpha, IL10, IL7, and IL2, as well as chemokines by respiratory epithelial cells, dendritic cells, and macrophages.57 According to recent reports, cytokine storm and severe inflammation are highly correlated for the progression of SARS-CoV-2 infection.58 It has been observed that lung epithelial cells play a crucial role in the release of several pro-inflammatory cytokines, including IL6 and IL8.59 Various inflammatory markers such as high levels of C-reactive protein, ferritin, and D-dimers as well as the neutrophil-to-lymphocyte ratio and serum levels of several inflammatory cytokines and chemokines are associated with the disease severity and death of COVID-19 patients.60 In addition, it has been observed that aberrant pathogenic T cells and inflammatory monocytes are rapidly activated, producing a huge number of cytokines and inducing an inflammatory storm.61
Nonsteroidal anti-inflammatory drugs (such as aspirin, ibuprofen, celecoxib, and indomethacin) are widely prescribed for the relief of pain and inflammation during COVID-19.62 These drugs alleviate inflammation through effectively blocking the production of prostaglandins via inhibition of cyclooxygenase enzymes.63 Anti-inflammatory therapeutics, such as colchicine, are used to treat COVID-19-associated excessive inflammation64 that acts by interacting with the Nod-like receptor protein 3 inflammasome protein complex, suppressing the release of the cytokines IL1β, IL18, and IL6.65 Colchicine also can bind to free tubulin dimers and block microtubule polymerization; therefore, it interrupts inflammatory cell activities and cytokine release.66 Moreover, colchicine is used to control the white blood cell-mediated inflammatory activities, thus inhibiting the production of superoxides and the release of numerous cytokines and pyrogens from the white blood cells.67 Quercetin and Ivermectin also have been suggested as anti-inflammatory drugs for the treatment of COVID-19 because they work by reducing the production of tumor necrosis factor-alpha, IL1, and IL6 as well as suppressing lipopolysaccharide-induced translocation of nuclear factor kappa-light-chain-enhancer of activated B cells.68 The signaling pathway of the anti-inflammatory effects occurs through the inhibition of enzymes like cyclooxygenases and lipoxygenases, which are responsible for eicosanoid generation. This in turn reduces the concentrations of inflammatory mediators such as prostaglandins and leukotrienes. Also, they prevent the activation of nuclear factor kappa-light-chain-enhancer of activated B cell target genes, such as those for the cytokines IL1α, IL1β, IL6, IL8, and tumor necrosis factor-alpha, which are considered potential markers for inflammation.58 Experimental studies indicate that C. speciosus has potent anti-inflammatory effects due to the presence of the active compounds costunolide and diosgenin, which have been isolated from the plant.12 The flavonoids and terpenoids present in the ethanolic leaf extract of C. speciosus also exhibit anti-inflammatory properties.34 To investigate the traditional uses of C. speciosus for the treatment of inflammation, numerous experimental studies have been conducted. The ethanolic extract of the C. speciosus rhizome has shown anti-inflammatory properties in carrageenen-induced edema formation in rats.35 In addition, the methanolic extract of the C. speciosus aerial parts has demonstrated anti-inflammatory activities in adult albino rats and Swiss albino mice.36 Likewise, the methanolic extract of C. speciosus leaves has shown anti-inflammatory activity by inhibiting the heat-induced hemolysis of red blood cells.19 The denaturation of proteins is one of the main causes of inflammatory disease. In-vitro studies have demonstrated that the ethanolic extract of C. speciosus leaves inhibits protein denaturation in inflammatory disease by controlling auto-antigen production.37 Moreover, the methanolic extract of C. speciosus seeds has shown significant anti-inflammatory activity by inhibiting hypotonic lysis of the erythrocyte membrane in a red blood cell membrane stabilization study. It also has been noticed that the methanolic extract exhibits anti-inflammatory activity by blocking cyclooxygenase activity followed by the inhibition of prostaglandin synthesis.38 Despite enormous efforts for the development of new drugs for the treatment of COVID-19, to date, no clinical trial has shown a validated significant effect. Therefore, there is still a lack of specific efficacious clinically proven drugs, vaccines, as well as other antiviral medications and therapies to control the virus that causes COVID-19.69 Based on the above-mentioned antiviral, anti-inflammatory, and immunoregulatory activity, C. speciosus might be a promising safe, natural candidate for the treatment and prevention of COVID-19 in the future.
Adaptogenic activity
Adaptogens are classical stress-protective compounds that help organisms to acclimatize under different forms of stress as well as to increase adaptability, resilience, and hence survival by activating certain adaptive signaling pathways of the cellular and organismal defense systems.70 Adaptogens also stimulate the cellular defense mechanisms and increase nonspecific resistance and adaptation to stress by expressing stress-activated proteins and activating the extra- and intracellular signaling pathways. Likewise, adaptogens coordinate to alleviate stress-induced mental and behavioral disorders.71C. speciosus rhizome extracts act as an effective adaptogenic agent when administered in stress-induced experimental albino rats. The extract showed antistress and adaptogenic activity in brain neurotransmitter profiles and in monoamine oxidase enzyme levels by normalizing norepinephrine, dopamine, 5-hydroxy tryptamine, and 5-hydroxy indole acetic acid against cold immobilization.39 This plant extract also has been used in the formulation of an effective antistress and antidepressant drug against central nervous system disorders,12 since the extract significantly reduced the stress-induced increase of 5-hydroxy tryptamine and 5-hydroxy indole acetic acid levels in brain tissues by preventing the alarm reaction, which causes a significant increase in 5-hydroxy indole acetic acid and 5-hydroxy tryptamine levels.40
Adaptogens play a potentially significant role at all stages of viral infections. They can decrease the duration of the acute phase of illness by affecting the neuroendocrine-immune system. Adaptogens likely combat infection through their specific and nonspecific antiviral properties, attenuate escalating inflammation through their potent anti-inflammatory effects, repair oxidative stress-induced injuries in compromised cells and tissues, and provide baseline support through their immunomodulatory, immunostimulatory, and anti-oxidant effects. In COVID-19 patients, the effects of adaptogens mainly occur during their convalescence. Adaptogens are primarily preferred for the treatment of viral infections as they modulate innate and adaptive immunity as well as anti-inflammatory activity, detoxify and repair the oxidative stress-induced damage in the cells, and direct antiviral effects by inhibiting viral docking or replication; thus, they can improve the quality of life of patients during convalescence. Recent data from clinical experimental studies have shown that melatonin, a novel therapeutic agent against SARS-CoV-2, helps to bolster the immune system, which is activated by adaptogens through the melatonin signaling pathway.72 Melatonin as an adaptive hormone73 also plays an important role in the regulation of homeostasis,74 which in turn can be activated using novel C. speciosus plant extracts.
Safe and nontoxic psychotropic medications that do not aggravate the psychiatric condition are a prerequisite for the treatment of COVID-19 patients.75 Therefore, considering the urgent need to find a specific pharmacotherapy for COVID-19 patients, the rhizomic extract of C. speciosus may be recommended as an easily available, affordable, nontoxic, and safe adaptogenic agent for the treatment of COVID-19 patients.
Antidiabetic activity
Diabetes mellitus is a chronic metabolic disorder caused either by the failure of requisite insulin production due to the loss of beta cells present in the pancreas (as in type 1 diabetes), or when the sensitivity of those cells is diminished due to insulin resistance (as in type 2 diabetes).76 Hyperglycemia occurs when an excessive amount of glucose circulates in the blood plasma; on the other hand, hypoglycemia ensues when the blood glucose level decreases. Both hyperglycemia and hypoglycemia may disrupt an already malfunctioning innate immune system in patients with diabetes, increasing their susceptibility to infections.77 Uncontrolled diabetes is associated with macro- and microvascular complications affecting the health and survival of patients.78
C. speciosus has been demonstrated to have immense antidiabetic properties as it regulates the secretion of insulin from pre-existing beta cells of the islets of Langerhans.11 This appears through induction of the expression of the insulin gene in pancreatic cells and insulin receptor-A in hepatic cells, and increasing the serum insulin levels consequently increases glucose uptake through induction of glucose transporter 2 gene expression. The hypoglycemic effect of various active constituents is exerted through potentiation of insulin synthesis and release from the existing beta cells as well as increasing the tissue sensitivity of insulin to glucose uptake. The leaves and rhizomes of C. speciosus have shown antidiabetic activity due to the presence of phytochemical flavonoids37 and diosgenin, a steroidal saponin.20 Additionally, costunolide, extracted from C. speciosus, significantly reduced the plasma glucose level when administered to streptozotocin-induced diabetic male Wistar rats at different doses41 by inhibiting the expression of nitric oxide synthase.79 Similarly, the administration of aqueous and methanolic rhizome extracts showed a reduction in the blood glucose level in streptozotocin-induced diabetic rats. The ethanolic extract of C. speciosus leaves was found to significantly reduce the blood glucose level when administered orally to diabetic experimental animal models.21 The ethanolic extract of C. speciosus rhizomes also showed a significant antidiabetic effect in alloxan-induced diabetic rats.42 In diabetic rats, the hexane crude extract of C. specious rhizomes was effective in bringing down and normalizing the serum glucose level.22 The in-vitro antidiabetic potential of C. speciosus was also assessed by a starch–iodine color assay.43
Most of the available clinical studies have shown that diabetes mellitus is one of the most common and prevalent comorbidities in COVID-19 patients that is causing considerable morbidity and mortality rates globally.80 Therefore, as C. speciosus is a potent antidiabetogenic agent, it should be considered as one of the first lines of defense. Patients with diabetes tend to have more severe disease vulnerability with poor glycemic control, which has a negative impact on the immunological system and may lead to a high risk of infections and serious life-threatening chronic complications with worse outcomes when they test positive for COVID-19.81In-vitro animal studies have shown that when angiotensin-converting enzyme 2 (ACE2), the main receptor responsible for COVID-19, binds with SARS-CoV-2, this biological mechanism reduces adequate insulin secretion by damaging the function of the beta cells of pancreatic islets.82 On the other hand, infection secondary to diabetic complications is also accompanied by a huge production of cytokines, causing cytokine storms, which may induce insulin resistance.83 Both altered insulin secretion and insulin resistance are responsible for uncontrolled blood sugar levels, which are generally noticed in patients infected with SARS-CoV-2; therefore, acute and chronic diabetes significantly affect both the innate and the adaptive immune system84 and are linked to poor white blood cell function, including impaired phagocytosis by neutrophils, neutrophil chemotaxis, macrophage and monocyte function, and innate cell-mediated immunity.85 Patients with diabetes (type 1 or type 2) have been identified as being at an increased risk for respiratory tract infections and serious illness from COVID-19 relative to the healthy general population86 because there is a defect in the innate immunity, which aggravates phagocytosis, neutrophil chemotaxis, and/or cell-mediated immunity.87
The mortality rate of COVID-19 patients with diabetes was significantly higher (∼42.3%) than that of COVID-19 patients without diabetes.78 Numerous recent studies have indicated that SARS-COV-2 infection can lead to multi-organ injuries with a worsening clinical status and composite adverse outcomes in individuals with pre-existing diabetes.88 In light of the currently available data, people with diabetes are at risk for developing severe and critical forms of COVID-19 and seem to be more susceptible and vulnerable; thus, they need special care.89 However, it is very obvious that patients with diabetes need to continuously control and regularly monitor their blood glucose levels, which requires careful clinical management, with extra precautions and special attention to prevent COVID-19. Therefore, it is urgently needed to develop novel antidiabetic medications, which will play a distinctive role in protecting people from infection and could reduce the risk of morbidity and mortality in patients with uncontrolled glycemia. Antidiabetic drugs (e.g., insulin, dipeptidyl peptidase 4 inhibitors, sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 receptor agonists, thiazolidinediones, metformin, sulfonylureas, pioglitazone, liraglutide, hydroxychloroquine, etc.) have been used in COVID-19 patients to manage their diabetes;90 however, they show various complications for lowering persistent high blood glucose levels. Therefore, in order to protect these patients, the administration of a drug formulated from naturally occurring C. speciosus may be prescribed as a future preventative therapy for patients with diabetes, as good glycemic control is the key to reduce the probability of contracting COVID-19 and will help to overcome the detrimental adverse effects of this disease. Due to its low cost, widespread availability, and good tolerability, C. speciosus plant extract is definitely a potential candidate and an appropriate add-on drug that could safely be prescribed as a sustainable, nontoxic, effective herbal antidiabetic drug therapy for treating and controlling the insulin levels of diabetic patients in order to decrease complications due to COVID-19.33
Antimicrobial activity
Co-infections, also known as dual-infections, secondary infections, or superinfections caused by multiple pathogens of viral, bacterial, or fungal origin, are a common complication causing severe illness among COVID-19 patients and result in a major risk of unfavorable outcomes, which may prolong the acute phase of COVID-19 and are also associated with increased rates of morbidity and mortality.91 Published clinical data show that COVID-19 patients are vulnerable to co-infections caused by bacterial and/or fungal pathogens.92 These bacterial and/or fungal secondary infections by multiple pathogens interfere with the immune status, which is a big threat to patients during COVID-19.93
Opportunistic infections by other microorganisms develop in patients diagnosed with COVID-19 during or after the initial infection with a virus, and they are associated with worse adverse outcomes than that of either infection on its own.94 Sometimes, due to the combined effects of the microbial pathogens, patients may be exposed to severe disease conditions leading to fatal clinical complications. The most common bacteria reported during co-infections in COVID-19 patients mainly include Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa, Klebsiella pneumonia, Acinetobacter baumannii, and Mycoplasma pneumonia.95 Secondary fungal co-infections (e.g., candidiasis, aspergillosis, cryptococcosis, pneumocystosis, and histoplasmosis) also have been identified in patients with COVID-19,96 including Aspergillus flavus, Aspergillus fumigates, Candida glabrata, and Candida albicans, among others.97 Mucormycosis, also known as the black fungus, is another serious fungal infection that produces life-threatening complications detected among COVID-19 patients.98
To overcome severe disease, various antibacterial, antifungal, and antiviral drugs are administered to COVID-19 patients.96 The use of antimicrobial drugs is one of the principal methods in medicine that is widely employed to help reduce and prevent different infectious diseases, either bacterial or fungal.99 The most frequently prescribed antibiotics that are used to prevent the occurrence of secondary bacterial infections in COVID-19 patients include ceftriaxone/cefotaxime, ampicillin/amoxicillin, quinolones, imipenem, ceftriaxone, fluoroquinolone, azithromycin, quercetin, rapamycin, doxycycline, fluoroquinolones, macrolides, cephalosporins, azithromycin, moxifloxacin, ceftriaxone, vancomycin, and cefepime.100 Meanwhile, the drugs used to treat fungal infections in COVID-19 patients include amphotericin B, itraconazole, caspofungin, fluconazole, and voriconazole.101
Some of the alternative antiviral drugs that are used in the management of COVID-19 target specific steps within the life cycle of SARS-CoV-2, such as darunavir, atazanavir, saquinavir (protease inhibitors); umifenovir (fusion inhibitor); remdesivir, favipiravir (inhibitors of viral RNA polymerase/RNA synthesis); lopinavir/ritonavir (inhibitors of viral protein synthesis); ivermectin (inhibitor of viral replication); hydroxychloroquine, chloroquine (viral entry inhibitors); oseltamivir (viral release inhibitor); nitazoxanide (imunomodulator); ribavirin, sofosbuvir (nucleotide reverse transcriptase inhibitor); emtricitabine, and azvudine (nucleoside reverse transcriptase inhibitor).102 As viral-bacterial-fungal co-infections are one of the largest medical concerns resulting in increased mortality rates, it is imperative to pay attention to these co-infections in critical patients positive for COVID-19.95 In most cases, patients are treated with broad-spectrum antimicrobial drugs with unknown efficacy103 for the suspected bacterial or fungal co-infections,104 but they are not very effective for the treatment of COVID-19 and also suppress the immune system.101 Based on the recent clinical data, serious adverse side effects have been reported in patients when treated with the above-mentioned antimicrobial drugs,92 since no specific drug has been approved and shown to be effective until now;93 whereas a newer drug development regime is time consuming107 for the prevention and treatment of human coronavirus infections.108 Therefore, immediate efforts and effective treatment strategies are needed to reduce the risk of transmission and to manage future pandemics.102 This critical reality demands urgency for a special focus on developing novel, effective, and safe antimicrobial drugs with more promising clinical development to combat invasive microbes.
From the thorough phytochemical studies and investigations of the available literature on C. speciosus, it has been clearly found that the C. speciosus plant extract possesses plethoric secondary metabolites (e.g., flavonoids, phenols, tannins, alkaloids, steroids, terpenoids, etc.), which act as an effective antimicrobial substance against a wide range of microorganisms.34 Hexane and ethanolic extracts of C. speciosus leaves and rhizomes exhibit promising antibacterial and antifungal activities against various pathogenic microorganisms.44 Moreover, the aqueous extract shows antibacterial activity against S. aureus,45 which is one of the causative agents for co-infection in COVID-19. In addition, it has been noted that the epoxidation of diosgenin extracted from the rhizome of Costus shows antifungal activity46 and that components of the essential oil (e.g., α-humulene and zerumbone) extracted from the rhizomes of C. speciosus possess considerable antibacterial activity.47
It also has been noticed that sometimes the use of synthetic antibiotic drugs increases the risk of death during secondary infections in COVID-19 patients.109 Furthermore, antibiotic overuse is associated with subsequent harm, which leads to resistance against the microorganisms,110 and these antibiotic-resistant infections are a significant threat to COVID-19 patients. On the other hand, it is very challenging to distinguish between severe COVID-19 and bacterial and fungal secondary infections.103
Therefore, the experimental results suggest that since the C. speciosus plant extract acts as an effective antimicrobial agent, it possibly could be used as a safe and effective antimicrobial medicine17 for invasive secondary bacterial and/or fungal co-infections, which are more life-threatening than the initial viral infection during COVID-19.
Anthelmintic activity
Anthelmintics are a class of generic drugs that are used to destroy parasitic worms without causing any significant harm to the host cell. Some reports of substantial anthelmintic activity of the methanolic and aqueous extracts of the aerial parts of C. speciosus against earthworms are present in the existing literature as they have been used widely for the primary evaluation of anthelmintic compounds in vitro because they mimic intestinal worms in their reaction to helminthiasis,48 suggesting that the plant can be used as a promising anthelmintic agent to cure the disease.15 There are plethoric anthelmintic medicines, viz. niclosamide, ivermectin, and nitazoxanides, in the market that are employed to symptomatically treat and cure COVID-19 patients.111 It has been reported that potential helminth co-infections exacerbate the immune systems susceptible to newer contagions.112 It also has been noted that helminth parasites have symbiotically adapted to their specific hosts during the long evolutionary process, thus resulting in chronic diseases of the host; however, the mortality rate is low. There are several modulations in the host surface that are brought about by these parasites; the immune system is one of them. In general, the parasites induce a hyporesponsive state of the host’s immune system, and, in situations like the COVID-19 pandemic, such attributes may worsen the situation per se. Therefore, the current situation demands the launch of newer therapeutic drugs and treatment options that are widely and easily available as well as affordable, effective, and safe, without causing any systemic side effects. As the C. speciosus plant extract has potent anthelmintic activity, it may be a potent addition to prevent SARS-CoV-2 viral replication and may be recommended for symptomatic treatment for further clinical evaluation and development of new drugs to cure and treat COVID-19.
Estrogenic activity
Estrogen, a primary female sex hormone that actively regulates the cellular and molecular processes, is involved in the development of the female reproductive system along with the maintenance of secondary sexual characteristics. Both ethanolic and methanolic extracts of C. speciosus rhizomes have been shown to exert an effective estrogenic effect in animal models.23,49 Additionally, uterine stimulant properties were observed when rhizome extracts of C. specious were administered on spontaneous phasic uterine contractions, which were mainly due to nonestrogenic effects with a consequent increase in uterotonic contractions indued via calcium entry on L- type calcium channels and sarcoplasmic reticulum calcium release.49 There are several reports explaining that the saponins present in the C. speciosus extract induced a profound estrogenic activity in sprayed rats, significantly increased uterine weight and uterine glycogen concentration, and produced proliferative changes in the uterus.50
Recently, estrogenic activity has been linked with the repair of infected respiratory tissues, which provide protection from developing a severe infection by SARS-CoV-2 in females and thus act as a strong immune-regulatory agent in both the innate and adaptive immune systems.113 It has been well speculated that the SARS-CoV-2 spike protein binds to ACE2 receptors and that estrogens play an important role in the interactions between the SARS-CoV-2 spike protein and the human ACE2 receptor that protects the human lung during infection caused by SARS-CoV-2.114 Derivatives of estrogen molecules (17β-diol and S-equol) modulate the ACE2-dependent membrane fusion protein and reduce the entry of the SARS-CoV-2 spike protein into lung cells. Estrogen also affects cardiac ACE2 levels and activity through its receptors estrogen receptor alpha and G protein-coupled estrogen receptor, by regulating ACE2 shedding via disintegrin and metalloprotease 17 and transmembrane serine protease 2. This linear correlation of ACE2 gene transcripts with estrogen receptor alpha and G protein-coupled estrogen receptor mRNAs is associated with higher values of ACE2 gene expression.115 Estrogens stimulate the immune system by modulating the function of B cells and thus improve T-helper 2 cell activity. They stimulate the nasal immune system by increasing the activity of phagocytes, dendritic cells, and natural killer cells.116 Estrogens are elevated during C. speciosus administration, which also exhibits anti-inflammatory and immuno-modulatory effects against COVID-19, thus reducing SARS-CoV-2 infectivity through modulation of pro-inflammatory signaling pathways.117
These observations strongly indicate the potential protective effect of C. speciosus via modulating estrogenic activity; therefore, it could be recommended for future therapeutic strategies against COVID-19 (Fig. 2).
Antipyretic activity
Cumulatively, antipyretics are a group of drugs that reduce fever during pyrexia or conditions leading to an increase in the core body temperature. The ethanolic rhizome extract of C. speciosus has been shown to exhibit an antipyretic effect in yeast-induced pyrexia in an animal model by acting through inhibition of the production of prostaglandins in the hypothalamus.35 An uncontrolled increase in body temperature leading to fever is one of the most common initial symptoms of COVID-19 in approximately 80% of patients,118 so it is considered that there is a close connection between pyrexia and the onset of the disease. Under these circumstances, it is mandatory to control and reduce fever with artificial antipyretics, which is beneficial for COVID-19 patients.119 Recent COVID-19 treatment guidelines by the World Health Organization have recommended the use of antipyretics24 as a first line of defense. Several synthetic antipyretics (e.g., ibuprofen, paracetamol, among others) are readily available in the market as over-the-counter medications; however, consuming them may lead to severe irreversible side effects.120 Therefore, the administration of C. speciosus extract or its derivatives for its natural antipyretic activity may be an effective option as a safe and nontoxic herbal antipyretic medicine for the symptomatic treatment of COVID-19 patients.
Conclusions
The therapeutic potential of C. speciosus extract or its derivatives against COVID-19 is significant, given their diverse modes of action and their ability to impact different cellular and molecular pathways that are related to the pathophysiology of the disease. The present review emphasizes the need for comprehensive studies to entirely understand the efficacy, safety, and mechanism of action of these natural compounds derived from C. speciosus. As the global scientific community continues to search for effective treatments for COVID-19, natural products offer a valuable resource for discovering novel antiviral agents.
In conclusion, the role of C. speciosus in combating COVID-19 is an area of great interest and potential. Continued research and clinical trials are essential to validate the efficacy of these compounds and to potentially integrate them into the broader strategy for managing and treating COVID-19. The exploration of natural products not only offers hope for new therapeutic options but also highlights the importance of biodiversity and natural resources in addressing global health challenges.
Abbreviations
- ACE2:
angiotensin-converting enzyme 2
C. speciosus :Costus speciosus
- COVID-19:
coronavirus disease 2019
- IL:
interleukin
- SARS-CoV-2:
severe acute respiratory syndrome coronavirus 2
Declarations
Acknowledgement
None.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Conflict of interest
The authors have no conflict of interest related to this publication.
Authors’ contributions
Conceptualization: AC; study design: DL; analysis and interpretation of data: DL; manuscript writing: DL, AC; statistical analysis: DL; administration: DL, AC; critical revision AC; technical or material support: DL; overall supervision: AC.

 Author information
Author information