Introduction
At least 25,000 years have elapsed between the artistic depictions of facial aging and scalp hair loss and the publication of clinically relevant cartoons illustrating patterns with severity grades (Fig. 1a–c).1,2 It is worth noting that more complex categorical systems3,4 emerged subsequently that covered a broader spectrum of clinical patterns. In our experience, simpler schemes shown in Figure 1b and c assessed the diversity of clinical impressions of pattern hair loss in a general dermatology practice, namely, subjective opinions without calibrated-quantitative estimates of hair growth and hair fiber formation.5 Indeed, miscategorization of hair loss can hinder the proper clinical assessment of known or novel treatments for pattern hair loss.
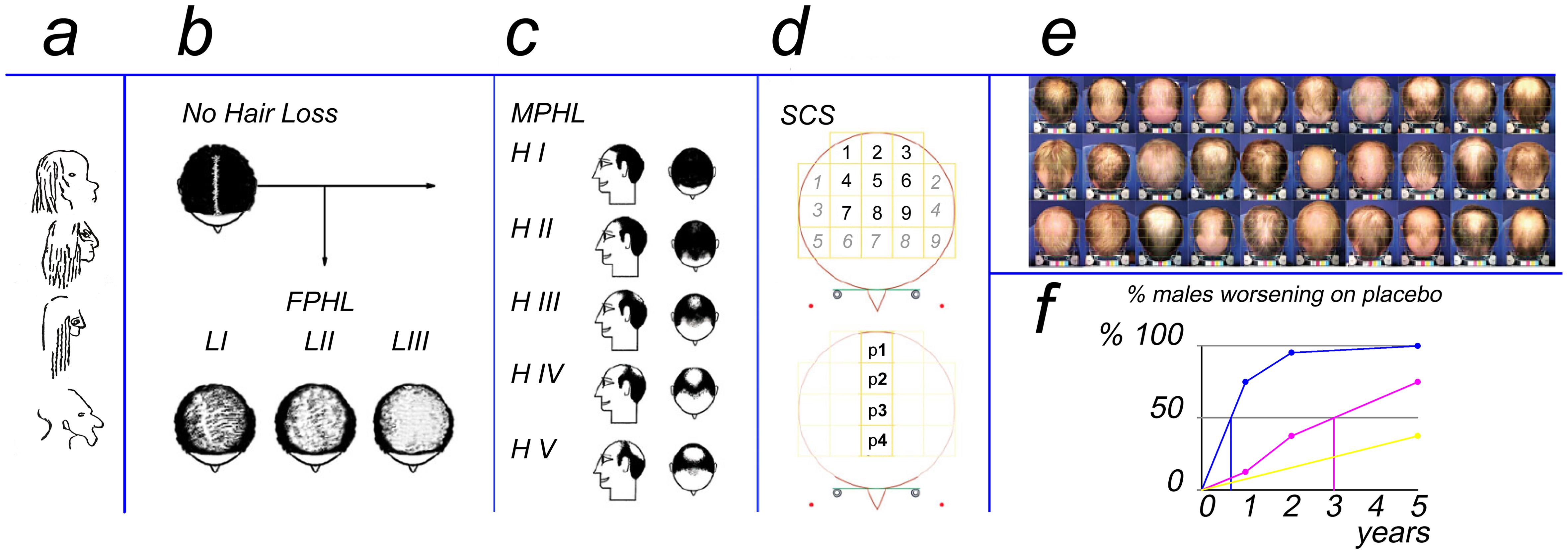
The subfigures present the evolution of hair loss imaging. (a) 25,000-year-old cave drawings (modified by the principal investigator with permission20). Cartoons of patterns along with severity grades for hair loss in females (b, FPHL2,5) and males (c, MPHL1,5) from 1960s to 70s. (d)The currently presented SCS method is a segmentation approach to estimate scalp coverage scoring independent of gender or pattern. (e) Standardized imaging of a panel of 30 male patients illustrates that cartoons do not always reliably fit clinically perceived patterning. (f) Precision in detecting regression using imaging varies by a method using the same sample of placebo-treated males with pattern hair loss (MPHL): clinical observation, yellow line, fails to detect 50% regression in 5 years; SCS assessment method detects 50% regression after 3 years (f. magenta line) while natural hair cycle regression was detected within 12 months if measured using phototrichogram (f, blue line). FPHL, females with pattern hair loss; MPHL, males with pattern hair loss.
At the turn of the 21st century, objective records with standardized global photographic approaches became a significant part of drug efficacy trials for patterned hair loss.6,7 However, during test-retest scoring of the severity of patterns on printed photographs, experienced clinicians reported a lack of reproducibility of the commonly used Hamilton-Ludwig scales.8 Finally, a 5-point scale was proposed for staging hair loss in females implementing the Venning and Dawber approach,9 a gender-restricted un-calibrated scale, applied in population studies. Accordingly,9,10 parting the wetted hair along the midline improved the ability to visualize scalp skin showing through the hair mass. Albeit expert consensus conferences generated guidelines,11 we found no formal guidance on methods for quantification of hair production and how to challenge questionable hypotheses like “regrowth of terminal hair from miniaturized follicles”.12
Published studies clearly illustrate shortcomings in terms of standardization of scalp preparation, hair growth parameters, and global imaging13–15 with low-quality images supporting efficacy claims in the patent literature.16
In the meantime, developments and innovations in hard and software imaging continue to progress. Essential steps require human intervention during imaging procedures and quality control of detailed hair growth analysis. For example, trichoscopy on the parted midline on the epicenter of patterned hair loss seemingly correlates with the severity scores taken as ‘hair mass’. The “Trichoscopy Derived Sinclair Scale”17 typically represents yet another indirect scaling procedure without real measurement of actual hair productivity. In this paper we raise the question: is there room for improved validation of a global method and can a non-experienced clinician be trained?
Historically we developed detailed exhaustive measurements of performances of individual hair follicles at the leading edge of the balding process. This detailed measurement was developed separately from more clinical global evaluations of scalp hair. The authors thought that it was timely to study correlations as both approaches, while quantitative and independently developed, seemed complementary. Before weaving the two methods into a unique continuum, we proceeded to the validation of the standardized dry hair approach for the practice of Scalp Coverage Scoring (SCS) in routine dermatology clinics and trials,18,19 we now propose that combing the dry hair with parting from the midline and segmentation of the top of the head into 4 square regions, from the crown to the frontal hair line, represents a promising and appropriate clinical development in the field. This applies to male and female patients with or without pattern hair loss and, consequently, SCS uniquely depends on differentiating scalp show-through from hair density in all hair loss patients independently of gender and hair loss pattern.20
While the SCS method has proven reliable and clinically useful in our hands, and phototrichogram methods are dependable when operated by expertly trained technicians and clinicians with access to digital imaging software, there remains the need for a simple and reliable calibrated scoring scheme for use in general practice. Herein we describe our success in transferring the SCS method to ‘naïve’ observers, regardless of their medical background, without the need for digital analysis equipment and with minimal training.
Material and methods
Scalp preparation and imaging
Improvement in scalp hair coverage assessment on parted hair beyond the use of cartoon scales resulted from precise estimates of scalp hair growth and productivity (a mix of density, diameter, and length) tested comparatively in the 504 subjects against SCS, as previously described.20 Hair productivity has been established by employing an original and validated method; the non-invasive exhaustive sampling method called contrast-enhanced-phototrichogram-with exogen-collection (CE-PTG-EC further PTG method). Productivity is a computation of [number of anagen hair per unit area (n/cm2)] × [diameter (µm)] × [linear growth rates (µm/day)]; the sum represents a fraction of the ROI-surface (ROI, region of interest) covered in a short period (usually 48 h). Another calculation expresses the time required to completely cover the ROI (e.g. 1 cm2).
Procedures for global scalp imaging, SCS, and PTG methods for hair growth dynamics on a small surface (around 1 cm2) are illustrated and compared in Figure S1. Classically, the region of interest was located at a distance from the epicenter i.e. in the progressing edge of alopecia (usually 4 to 7 cm from the midline on the top of the head) as outlined by a ring or identified with a micro-tattoo dot on clinical photographs in this particular comparison, like during previous clinical trials.20–22,26 With this unique approach, readers can independently discern what might seem ‘theoretically’ obvious to investigators, clinical research organizations (CROs), and industrial companies, yet it continues to be a subject of debate in regulatory agencies (ex: FDA, EU). This method may be the only way to truly appreciate the correlation—or lack of it—between the global perception, the SCS, and the detailed hair productivity measurements (Further details on the comparisons between methods are presented in Figures S2 and S3 with a historical record providing more detailed aspects of the figure in File S1).
The question of combining clinical severity and scoring methods was resolved as follows. We developed a segmentation approach to estimate scalp coverage scoring (SCS) as an objective global method (Fig. 1d). Scoring precise areas that cover the top of the head or vertex helps quantify loss with or without patterning independently of gender.18,19 Indeed standardized imaging illustrates that real patients do not always fit simplified classifications (Fig. 1e).
A speculative and experimental SCS assay based on a 5-year follow-up of untreated males with patterned hair loss (Fig. 1f, placebo) suggested that 50% of subjects would be rated as worsened in around 3 years (Fig. 1f, magenta line). The threshold would be reached after more than 5 years if changes were based only on clinical impressions without precise tracking (Fig. 1f, yellow line). Close-up imaging as with PTG required less time to detect regression of productivity i.e. less than 1 year (Fig. 1f, blue line).20–22 Tentatively, more precise hair productivity measurements might anticipate trends to regression in hair loss sufferers.
A longer TTCC indicates reduced hair productivity and decreased future growth potential in more severely affected patients. In contrast, less affected subjects with higher productivity (and faster growth rates) show reduced time to complete hair coverage and herald better prognosis in terms of responses to treatments. In essence, TTCC encompasses all measurable dynamic criteria.
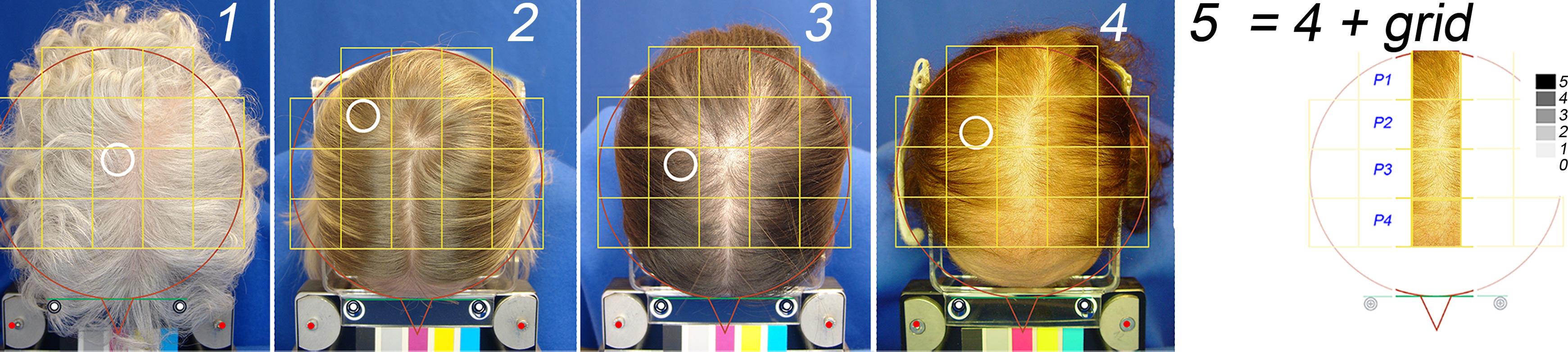
Therefore, we proposed combining SCS with detailed hair productivity by the PTG method. Standardized scalp preparation protocol was as follows: before they visited the clinic, patients were instructed to wash their hair at least twice weekly, with the last shampoo on the morning of the assessment. Upon arrival at their appointment, patients presented with dry hair, free of any extraneous materials, and preferably unclipped and unshaved (at least 4 cm long). Initially, scalp hair was combed in a wheel’s spokes pattern as subjects entered the examination room, which was illuminated with fluorescent light. With assistance from the technician in charge, subjects positioned their heads correctly within a stereotactic device that delivered light at a right angle23 to the top of the head. Finally, the hair was parted along the midline, and a new image was captured according to the protocol (Fig. 2).
Panels 1–4, global views of the top of the head (4 representative patients) show clean dry scalp hair parted on the midline overlaid with a grid. The white empty circle shows the region of interest (ROI) where hair productivity was measured using PTG. From left to right (numbers 1 to 4), the principal investigator qualified subjects as no-pattern, Ludwig I, II, and III. The latter is further illustrated with the 4 fields along the midline (panel 5: p1 to p4). The gray scale in the upper right corner of panel 5 is a symbolic representation of 5 degrees on a ‘numeric difficulty scale’ to see scalp skin through the hair (black (5): very difficult; white (0): no difficulty). ROI, region of interest; PTG, phototrichogram.
SCS training, transfer of competence, and testing of evaluator performance
SCS: basic training and developing the evaluation criteria
A series of clinical cases, unrelated to the present assay, were identified and images were provided as a training set. Whenever necessary, the principal investigator delivered extra instructions providing up to 380 fields with SCS data to support naïve observers on how to score with the SCS method.
Methodological key points were explained by the Principal Investigator using a privately owned training package (PowerPoint slideshow). Various examples of clinical situations and potential confounders were analyzed including but not limited to the skin of color linked mainly to ethnic background, the contrast between phases i.e. dark or white hair on paler or darker skin, the influence of various external interventions i.e. hairdressing and styling, imaging on parted clean or wet and dry scalp, lighting conditions, as indicated in detail previously.23 Observers were instructed to consider scalp skin and the mass (density) of hair as 2 separate phases: a discontinuous phase made of individual hair fibers and a continuous phase representing the skin underneath the hair mass.
After completing the short training (PowerPoint presentation with a series of clinical cases with SCS values determined as “a ruler” by the principal investigator), the observer was capable of estimating how easy or difficult it was to see the continuous phase i.e. the scalp skin through the hair. Not focussing on the amount of hair in the discontinuous phase was a fundamental requirement for the training and would prevent a cognitive bias. For this, a symbolic ‘difficulty’ scale was developed and shown simultaneously with the parted midline as a 5-point grey scale (Fig. 2, panel 5). In this scale white-transparent field was equal to a score of 0 and in the dark-grey or black field a score of 5. Accordingly, a score of 5/5 means it is ‘almost impossible, or very difficult to notice scalp skin’, while 0/5 means ‘no difficulty seeing the skin through the hair’, within the field of interest. During the scoring exercise observers were shown the global aspect (Fig. 2, panels 1 to 4) together with the 4 fields to be scored (exemplified for patient 4 by combining top view and segmentation grid as shown in panel 5). The statistical analysis was performed after the data were encoded and subsequently correlated with individual statistics (see Table 1).
Detailed clinical data and hair parameters of 10 selected test subjects
| Clinical Criteria | Hair number per unit area (n / cm2) | Anagen (Diameter µm) | Productivity | Cluster n° | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Graph | Age (years) | Category | Exogen Total | Nanos | 20–30 µm | 40–50 µm | ≥60 µm | n | % | TTCC | SCS % Max | Rank TTCC |
| Female | Blue | 38 | L I | 3 | 25 | 46 | 190 | 228 | 200 | 88% | 21 | 93 | 1 |
| Female | Blue | 78 | Other | 2 | 26 | 19 | 15 | 156 | 152 | 97% | 25 | 90 | 1 |
| Female | Blue | 48 | L III | 9 | 45 | 36 | 36 | 82 | 63 | 77% | 49 | 60 | 2 |
| Female | Blue | 24 | L II | 11 | 83 | 91 | 94 | 53 | 46 | 86% | 56 | 60 | 2 |
| Male | Red | 30 | H III | 1 | 14 | 24 | 77 | 117 | 82 | 70% | 54 | 75 | 3 |
| Male | Red | 23 | H III | 1 | 61 | 48 | 86 | 149 | 68 | 46% | 53 | 73 | 3 |
| Male | Red | 26 | H III | 5 | 29 | 94 | 108 | 83 | 51 | 61% | 56 | 70 | 4 |
| Male | Red | 30 | H IV | 18 | 82 | 81 | 95 | 76 | 55 | 72% | 66 | 55 | 4 |
| Male | Red | 48 | H V | 13 | 62 | 85 | 118 | 72 | 38 | 53% | 95 | 30 | 5 |
| Female | Blue | 60 | L III | 26 | 241 | 111 | 16 | 33 | 29 | 87% | 122 | 48 | 5 |
Summation of the scores recorded on the 4 different fields covering the midline (P1–P4 as shown in Figs. 1d and 2, panel 5) generate a maximum total score of 20 in case of complete coverage i.e. great difficulty in seeing any scalp through the hair. The sum was converted into a percentage as follows: (4 × 5) / 20 equals 100%. Conversely, 0/20 means ‘no difficulty at all to notice scalp skin’ i.e. 0% in relative terms. The SCS% of maximum (SCS% Max) was further processed statistically when comparing the scoring consistency between the Principal investigator and the newly trained observers.
SCS selection of 10 test cases
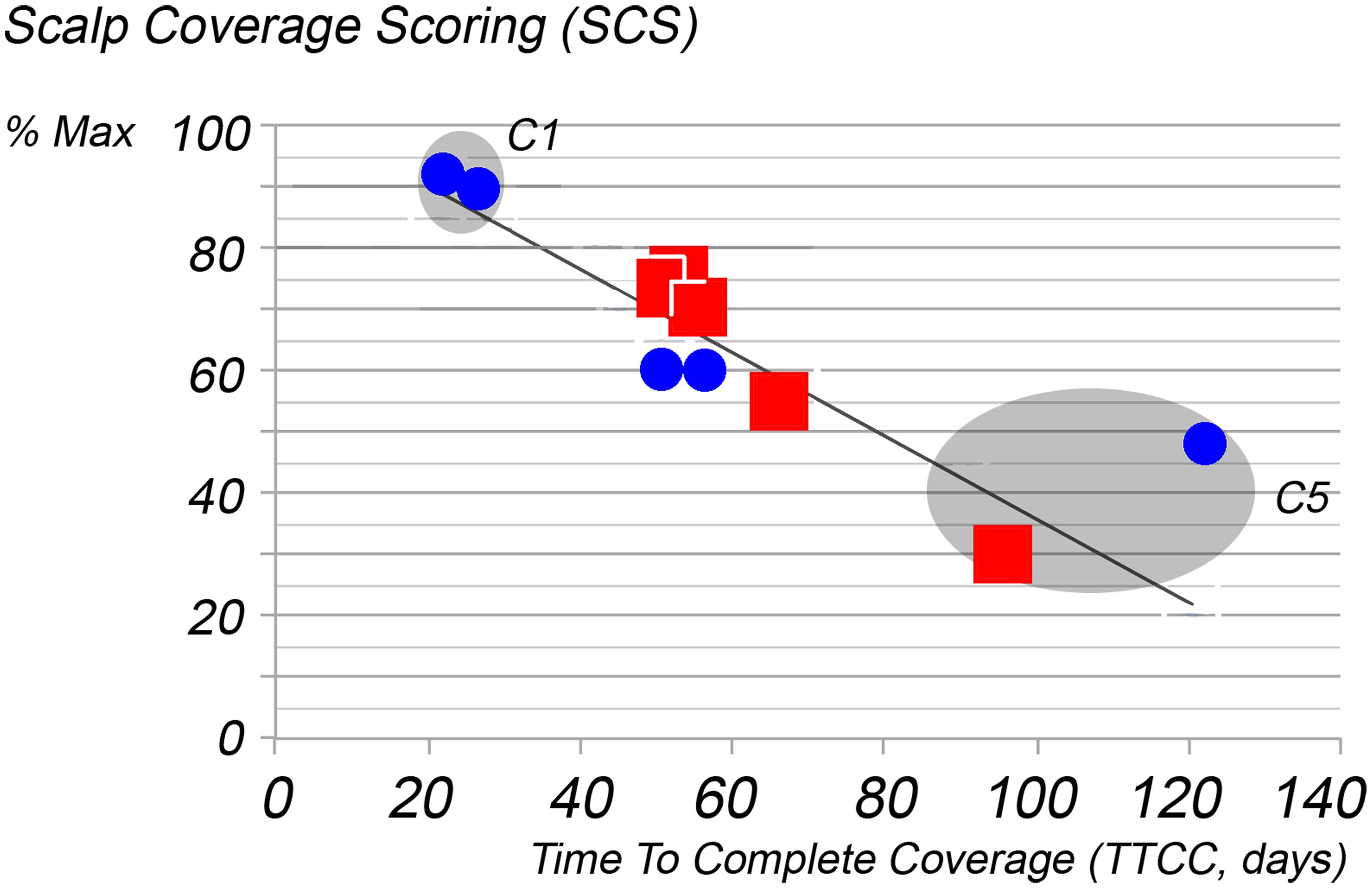
The principal investigator screened the Skinterface-clinical-database. Best quality images (2,592 × 1,944 pixels; 72 DPI reset to 41 × 36 cm for inclusion in PPT files) from 10 subjects complaining of hair loss, 5 males and 5 females with moderate to high hair density, were selected. All were scored by the principal investigator and cases were plotted along with PTG-measured productivity on the Hair Mass Index (HMI) abacus (Fig. 3). The 10 images to be used for the training set were chosen as close as possible to the experimentally validated regression line i.e. Hair Mass Index abacus. This HMI mathematically interrelates the scalp coverage score and the time required to completely cover the region of interest (TTCC), using a population of 504 subjects as detailed elsewhere.20 TTCC is the number of days taken by growing hair in a clipped area to cover the area completely.20 In short, more days in TTCC means less productivity including a possible mix of less hair, smaller diameter and/or lower growth rate. SCS values are expressed as the maximum (% Max) that could be reported i.e. 100% = 20/20 and 0% = 0/20. The hair productivity along with SCS%Max of our test sample (x-y coordinates of 10 selected patients; females: blue dots; males red squares, Figure 3.) appear all close to the regression line known as the Hair Mass Index abacus (HMI). The HMI-regression line represents the population statistics (see Figure 4) as established earlier on 504 test subjects and around 500,000 hair measurements.20 The x-y coordinates of individual patients were grouped 2 by 2 in 5 clusters. Clusters contain the closest neighbors in terms of TTCC.
Correlation of time to complete coverage (TTCC), namely, hair productivity data in our test sample (x-y coordinates of 10 selected patients; females: blue dots; males red squares) against the relative scalp coverage scores (SCS % Max; y-axis) established by the principal investigator. Closeness to the regression line known as the Hair Mass Index abacus (HMI) reflects the alignment of SCS scores with data established earlier on 504 test subjects and around 500,000 hair measurements.20 C1 and C5 are two clusters reflecting good (C1) and poor (C5) scalp coverage. SCS, scalp coverage scores; TTCC, correlation of time to complete coverage.
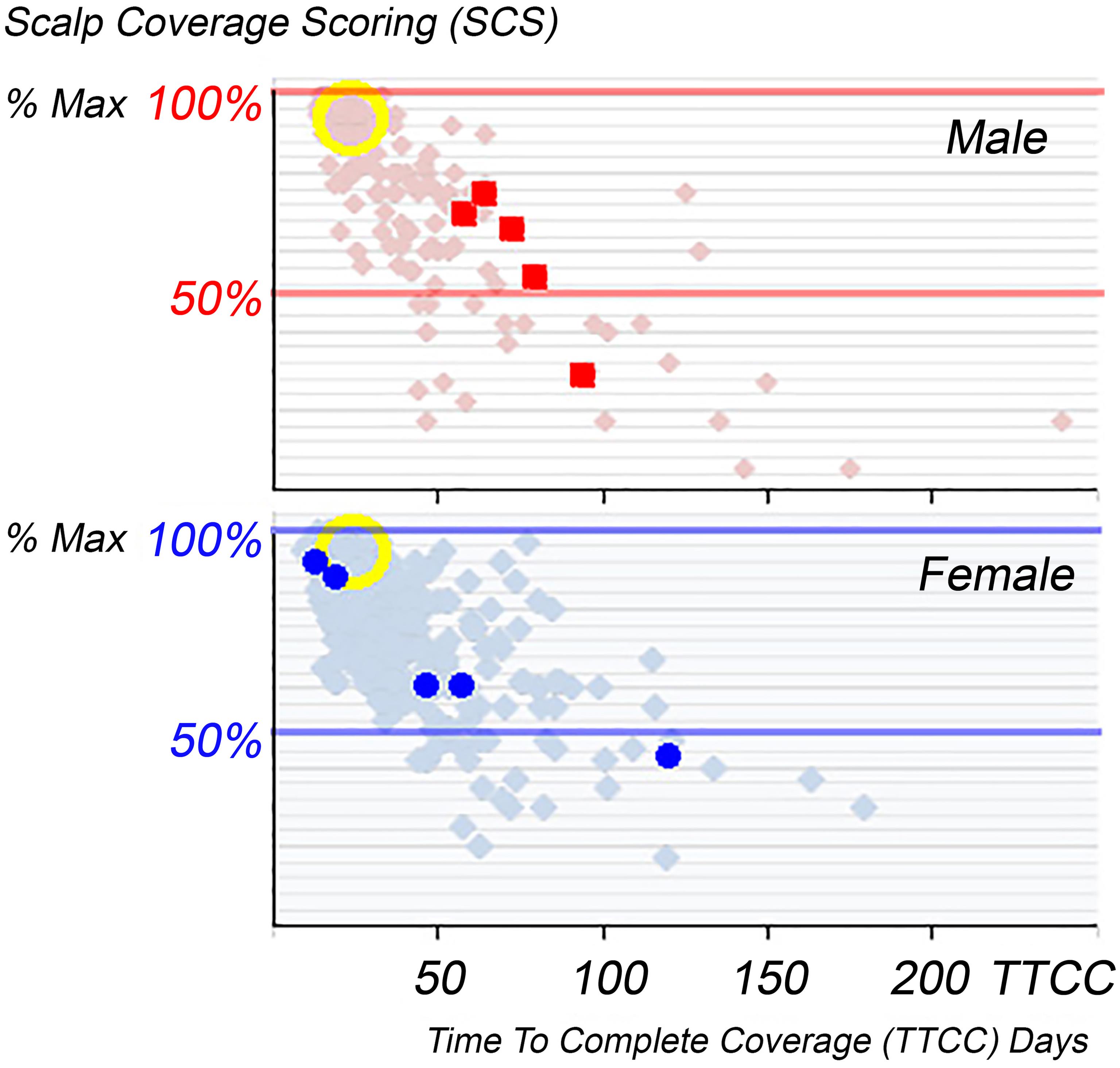
Productivity appears on the x-axis as Time To Complete Coverage (TTCC; days) as in Figure 3 against Scalp Coverage Scores (SCS) by the principal investigator expressed as the maximum (% Max). The hair productivity of selected test subjects i.e. 5 males and 5 females (respectively top panel red squares and bottom panel blue dots) were plotted individually against 414 patients’ data i.e. 110 males and 304 females - all complaining of excessive hair loss. The yellow circles highlight normal control data (90 test data) with high SCS and short TTCC. The individual clinical data and PTG statistics are ranked according to TTCC as detailed in Table 1. SCS, scalp coverage scores; TTCC, correlation of time to complete coverage.
The SCS and hair productivity in the 10 selected cases ranged respectively from a maximum of 100% down to less than 50% coverage while the productivity expressed as TTCC in the region of interest was almost equal to normal (cluster 1; 21–25 days) as opposed to 5.8 times longer (TTCC: 95–122 days in cluster 5) i.e. almost no potential for complete coverage as compared to normal controls (detailed in Table 1).
Images were randomized in a PowerPoint format, numbered 1 to 10 and no additional information (such as gender, clinical history, SCS, or productivity) was available to any observer before their involvement in the present scoring assay. The principal investigator also participated under the same conditions using the same PowerPoint slideshow and scored the SCS four times spread over a week with records blinded during the repeat days of SCS performance. The first test-retest session occurred in 2020 and the second one in 2022. Overall, with four sites per subject, the principal investigator conducted 320 evaluations.
SCS by trained observers
The training was delivered to 14 observers, 4 without medical background (trained via remote e-sessions; Belgium, France, and UK) and 10 clinical Dermatologists (face-to-face in one session) employing a PowerPoint slideshow. Their medical/dermatological training took place in different European countries (Belgium, Bulgaria, France, Netherlands, Portugal, and Ukraine). The former 4 tested SCS at their offices while the latter 10 attended a live session together in Belgium (Spring 2022). After due training, each observer received a complete set of 10 numbered randomized images without any identification of severity, gender, etc. They undertook SCS evaluations, exclusively on the 4 fields (P1–P4 as shown in Figure 2) with parted hair along the anterior-posterior axis. Cumulatively 560 scores were collected by observers.
SCS data collection and processing
Scores were collected anonymously by the investigator and processed for statistical analysis.
Statistical analyses
The individual source data i.e. clinical and analytical records appears in Table 1.
SCS values were calculated as % of maximum coverage on a per-patient basis.
Observers were categorized into 2 groups: one without (n = 4) and another one with a medical background (n = 10). Analysis of variance compared scores by these 2 panels vs scores by the principal investigator who rated SCS on 4 different days in 2020 (Statview®; Scheffe F-test; Bonferroni/Dunn) to test inter-observer variations. Analysis of variance (ANOVA) was also used to compare the average of 4 readings made by the principal investigator in 2020 with those made in 2022 to test intra-observer variation. In case variations would not be statistically significantly different, regression correlation was evaluated. Finally, hair loss patients were clustered 2 by 2 into 5 clusters as detailed in the last columns in Table 1 i.e. according to productivity i.e. Time To Complete Coverage (TTCC). These clusters will appear on the HMI abacus.
Results
Clinical Severity vs SCS
From the clinician’s perspective, SCS quantifies deviations from normal phenotype (Fig. 2, Table 1) and correlates with scalp hair productivity (measured as time to complete coverage; TTCC). The latter, as well as the former, were only partly reflected by static parameters like different types of hair densities,20 as confirmed in the present assay (Table 1, Fig. S2). For completeness in Table 1 we give threshold values for 5 essential parameters in female and male test subjects (F; M) as deviations from percentile 95 in our controls as published extensively:20 exogen: F: 14, M: 7; nano F: 17/cm2, M: 19/cm2; terminal hair F: 228 cm2, M: 241 cm2; TTCC: F: 31 days, M: 34 days; and SCS always over 95%. During the COVID-19 pandemic, basic training and the development of the evaluation criteria were given at a distance and the four observers practiced the SCS method at leisure in their own offices. The live session with 10 Dermatologists combined training with scoring exercises in one single session (May 2022).
Regarding the clustering, individual data for all subjects beyond cluster 1 exhibited abnormalities for most parameters. Interestingly, neither terminal hair density nor anagen hair (absolute density or relative percentage) perfectly corresponded with hair productivity or its clinical manifestation, and scalp coverage scoring. To better understand the clinical implications concerning the statistical analysis, we typically grouped the ratings of the two females shown on the left (subjects 1 and 2 in Fig. 2) in cluster 1, categorizing them as less affected, while the most affected female (Ludwig III, subject 4 in Fig. 2) was grouped with a male (Hamilton V) categorized as cluster 5.
SCS: transfer of competence and testing of performance
The typical global appearance of scalp coverage is exemplified in 4 females (Fig. 2). While the observers had no access to detailed clinical data and hair productivity parameters, all 10 cases published herein were chosen on the basis first of their source value of SCS confirmed by hair productivity or TTCC values. Transfer of competence was deemed successful as these 2 combined parameters were quite close to the regression line of the population HMI-abacus as established previously.20 The details of selected cases are described in Table 1 along with their nearest neighbor approach constituting 5 clusters of SCS values.
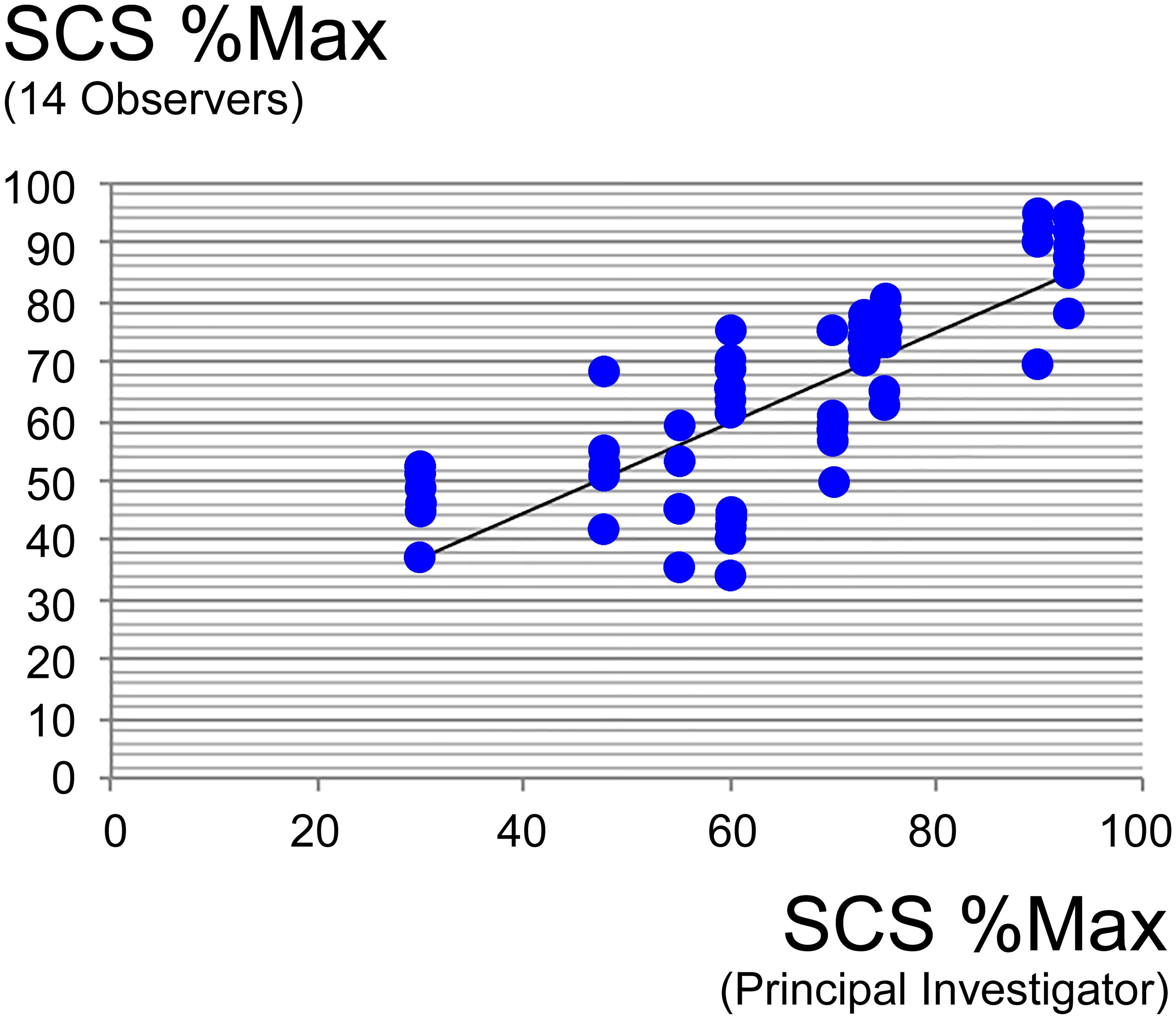
After reporting the individual data (Table 1) and placing the selected cases and clusters on the HMI abacus (Fig. 3), the combined SCS and hair productivity results of five male and 5 five female subjects were also displayed separately. These results were contrasted against the individual data of all our patients and volunteers, thus establishing our ‘hair loss population’ of 342 females and 162 males. These comparisons are displayed in Figure 4.
SCS: comparative performance of trained observers and principal investigator
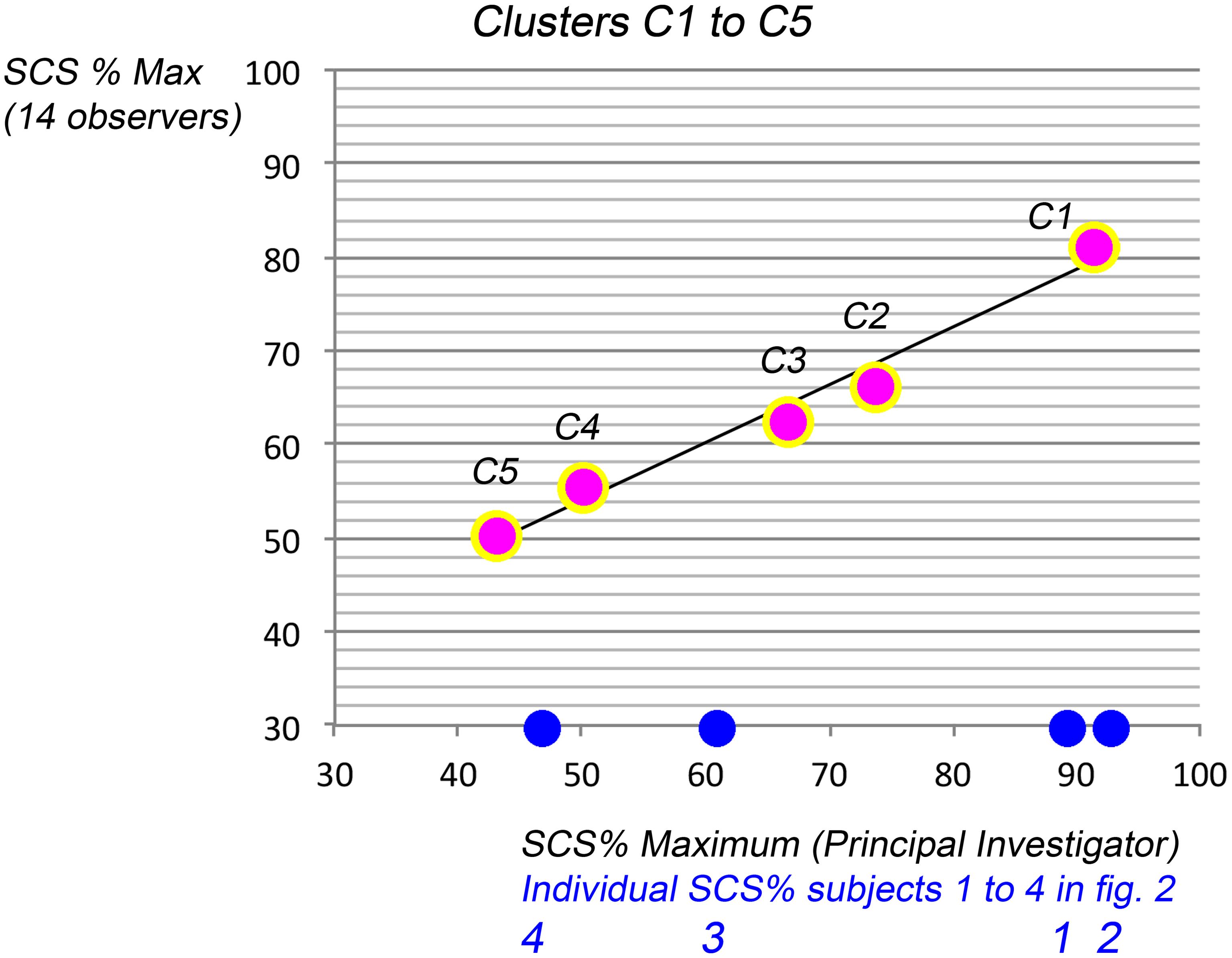
Analysis of variance detected no statistically significant differences between scores by the adequately trained observers versus the principal investigator (ANOVA). The trend of correlation (Figs. 3 and 4) was confirmed after clustering with an excellent linear fit (Fig. 5). Trained observers scores correlated linearly with those of the principal investigator (R2: 0.975; equation: y = 23.22 + 0.62 x). Hence, the SCS method seemed easily transferrable to a researcher, even with remote training, and their scoring equaled SCS by the principal investigator.
The 5 data points express the percentage of maximum (y-axis; SCS % Max. by 14 duly trained observers) meaning from top to bottom the least affected subjects (over 90% hair coverage) to the most severely affected ones (between 30 and 48% of SCS Max.% i.e. severe hair loss). The observer’s 5 data points were compared with scores proposed by the Principal investigator (x-axis; SCS%, PI). For the sake of completeness, the blue dots on the x-axis relate to the 4 females in Figure 2 with SCS given by the PI. Overall, there was a linear fit between trained observers and the PI (R2: 0.975) with a linear equation: y = 23.218 + 0.6165x. SCS, scalp coverage scores; PI, principal investigator.
SCS: inter-observer variability and intra-observer repeatability
As the statistical approach includes acceptable variation and known errors, we also detail inter- and intra-observer variations. During 2 test-retest sessions (4 days each over 1 week - 2 years apart), the principal investigator appeared to deliver consistent SCS values with a regression line y = 15.99 + 0.82 × (R2 = 0.92). Although there were some inter-individual variations, there was a linear fit between 14 trained observers and the principal investigator according to the following equation: y = 14.2 + 0.76 × (R2: 0.62; Fig. 6).
The average scores by 14 trained observers express the percentage of maximum (y-axis; SCS % Max) against average scores of 4 times repeat sessions made in 2020 by the Principal Investigator (x-axis; SCS% Max). Albeit inter-individual variations, there was a statistically significant linear fit between 14 trained observers and the principal investigator (R2: 0.62) with a linear equation: y = 14.2 + 0.76 x. Comparatively intra-individual variation was less as SCS by the principal investigator in 2020 and in 2022 equated as follows: y = 15.99 + 0.82 × (R2 = 0.92). Strict consistency studies with repeat imaging over time and tracking confounders are illustrated in Figure S3. SCS, scalp coverage scores.
Discussion
The primary methodological finding of this study is that ‘scalp coverage scoring’ (SCS) is an easy-to-use and clinically relevant method for estimating scalp hair loss. Furthermore, the SCS method can be quickly mastered when training sessions are conducted face-to-face or via remote technologies. After appropriate training, the performance of ‘naïve’ observers statistically matches that of the principal investigator who has 20 years of experience using this method. Notably, neither the observers’ general educational background nor their prior detailed knowledge of hair assessment affected the outcomes.
From the conception of SCS, we chose to avoid examining paired images as before-and-after pictures. By ensuring similar exposure, SCS on images viewed individually addressed the issue of time-related bias.18,19,25
The scalp and hair preparation is key for reproducible imaging and scoring as well during the initial consultation and for follow-up studies, as stressed by several groups.18–26 This report highlights the clinical importance and relevance of using SCS on standardized global scalp images and that it is independent of other parameters like the specific scalp area involved. In short, SCS is an objective rating of severity, independent of time, gender, and pattern offering quantitative assessment from global images. The SCS method, unlike others,1–5 does not include the frontal hairline that remains key for clinical identification at first glance of patterned hair loss, especially in females where it is generally – more or less well-retained.2 We developed this approach for images taken of the top of the head as the curvature is rather small in this field which flattens the image and aids analysis. Others have pointed out the difficulty of assessing global top-of-head images.8,23
During the initial stages of this research and development, we anticipated that the scalp’ relationship with the frontal skull curvature could present challenges, leading us not to develop this technique for alopecias primarily involving the frontal hairline as in frontal fibrosing alopecia. Standardizing the combing or styling of the hair at the front for top-of-head imaging also poses challenge. Additionally, the hairline often presents a binary situation—either hair is present or not across the margin of the hairline—both making it more difficult to assess the percentage of the area involved. The frontal line is important for the severity classification of the pattern of hair loss. It can be subjectively incorporated into the SCS score by the clinician. Moreover, our cohort comprises females with hair loss but without evidence of patterning, and – by definition - none had inflammatory conditions.
Furthermore, as most patients underestimate the severity of their condition at baseline,20 we advise not to share images with the patient. Showing these views to the patient during follow-up studies might become a confounder. During clinical trials, the sharing of images remains to be tested in terms of increasing or decreasing anxiety.27,28
Using cartoons as a guide for severity scoring of patterned hair loss is the ‘norm’ in clinical practice and quite efficient for diagnostic purposes. However, the use of this cartoon representation of hair loss patterns lacks reproducibility and sensitivity across time (personal unpublished experience).8
In a recent study, we drew to the attention of hair researchers that the intervals between clinical categories or severity classes as depicted in the cartoons, were unequal.20 With few exceptions, unequal intervals e.g. severity grades 2 and 5 may not be averaged as grade 3.5 while a progressive recovery towards normal pre-pubertal phenotype or productivity might remain an ideal target. It remains beyond this paper to discuss all biological reasons related to this yet undefined threshold but hard evidence favoring the hypothesis of partial ‘irreversibility’ has been published.20–22,29–31
Clinicians will probably see a percentage of patients complaining of hair loss with a substantial mass of hair and SCS max 100%. These may be the initial steps towards moderate telogen effluvium29–31 with a significant amount of hair shedding. Phototrichogram, unit area trichogram, and trichoscopy methods can detect some degree of hair follicle miniaturization with a significant increase of miniaturized fibers (less than 40 µm) or extreme thinning (less than 20 µm diameter). As growth rate together with the duration of anagen are subsequently drastically reduced, such short anagen hair follicles enter telogen prematurely and appear on the scalp with very short fibers. Once quantified, the presence of increased exogen, nano hair, and thinning in the 20–30 µm diameters (see Table 1) support the distinction of initial steps of reduced hair productivity on the way to patterned hair loss while coverage is still close to a normal phenotype.
From the more global perspective, the human eye, even in observers with a large clinical experience, has a preference for a limited scale for differentiation (e.g. 5 categories) and 20% differences are considered as a reasonable limit of precision for clinical estimations.29–32 While nothing impressive occurs at a distance from the frontal hairline with incipient patterns (Hamilton I-II) we previously showed that in more severe situations (Hamilton V, Ludwig III) coverage reduction by 20% represents a significant decrease in hair growth/density along with a dramatic increase in the time to complete coverage,20 thus sensitivity in detection of early hair loss is reduced.
Conclusion
Thus, SCS as a global and clinically relevant quantification approach for assessment of scalp coverage by hair seems to operate well after a short training session and has reasonable sensitivity and accuracy. The method is simple enough to be applicable (in real-time) in the medical office.
Limitations of the SCS method lie in the repeated reproducible preparation of the patients’ hair before imaging (styling and combing on the midline) and consistently reproducing this preparation on each visit. Whatever human intervention, there might be a methodological drift over time (examples are shown in Figs. S1 and S3). This being taken into consideration, SCS appears as the most relevant forerunner to validate artificial intelligence approaches using images for the assessment of hair thinning and great care should be taken with interpretation of coverage in the absence of precise measurements of hair productivity. Applicability of the HMI-abacus should be restricted to chronic, slow hair regression phenomena i.e. clearly not inflammatory conditions like alopecia areata or chemotherapy-induced loss (details can be found in File S1 and Fig. S1).
In summary, we propose SCS to the hair clinician and industry hair researcher; its simplicity, its translational potential, its use of standardized and well-controlled imaging of the top of the head, and the ability to bring it together with other methods such as PTG (phototrichogram), make it a valuable addition to the armory of methods for clinical hair research.
Supporting information
Supplementary material for this article is available at https://doi.org/10.61474/ncs.2023.00014 .
Fig. S2
Statistical approach and rationale for selecting global and analytical criteria.
(DOCX)
Fig. S3
Tracking confounders when Scalp Coverage Scoring is employed during longitudinal studies.
(DOCX)
File S1
A short note on history from exhaustive hair growth measurements to calibrated Scalp Coverage Scoring and Hair Mass Index.
(DOCX)
Declarations
Acknowledgement
In the method testing, Local Group of Dermatologists (Groupe Local d’Evaluation Médicale, GLEM, Tournai; Belgium with members: Bataille S, Dimitrova G, Duchatelle A, Ferreira B, Gordieieva M, Herbaut D, Mathieu A, Olemans C, Vandeweghe B.) participated in a one-day collective live face-to-face training and scoring session to support us. The Research on SCS-HMI-PTG has been awarded the “DermImpact Award” sponsored by J&J-Janssen (Belgium). The award was attributed to the Principal Investigator under the auspices of the Royal Belgian Society of Dermatology and Venereology (SRBDV-BVDV; on the occasion of the yearly meeting held in Brugge, on March 23, 2023).
Ethical statement
The study adhered to the principles of the Declaration of Helsinki. All methodologies, clinical images, and study protocols were approved by the local Ethics Committee. The samples for tutorial and testing did not involve new patients or volunteers. Written informed consent was obtained from each participant.
Data sharing policy
The supplementary files are published online together with the article.
Funding
None.
Conflict of interest
The authors declare that there are no conflicts of interest
Authors’ contributions
Clinical and scientific development of innovative technologies: DvN and VR; writing and project administration:DvN; manuscript draft and editing: HR, GW, LD and DvN; manuscript review: HR and GW. method testing: HR, GW, LD, VR and KR. All the authors were actively involved with their work on this manuscript and have approved the final version of the manuscript.

 Author information
Author information