Introduction
Melanoma is the most fatal skin cancer. As a result, melanoma treatment has been the focus of numerous preclinical and clinical investigations aimed at further understanding cancer immunobiology and evaluating different tumor immunotherapies.1,2 Over time, targeted therapy and immunotherapy with checkpoint inhibitors have changed the course of this disease.3
In 1992, when screening genes involved in apoptosis, the team of Tasuku Honjo reported the discovery of a protein, which they named programmed death-1 (PD-1), expressed on the surface of a subset of immune cells known as T cells.4 In 1999, a ubiquitous antiapoptotic receptor on cancer cells was reported from the Mayo Clinic.5 Initially named B7-H1, it was later renamed PD-L1after being identified as a ligand of PD-1. In 2002, Iwai et al. reported that blocking the interaction between PD-1 and its ligand enhanced immune activation, resulting in significantly stronger antitumor responses.6 Based on these findings, a fully human anti-PD-1 antibody was developed in 2005 by Ono Pharmaceutical Co. and Medarex (later acquired by Bristol-Myers Squibb). The development of therapeutic anti-PD-1/PD-L1 monoclonal antibodies, which induce the reactivation of specific antitumor immune responses, has emerged as a promising strategy for cancer immunotherapy.
First reported in 1987, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) was later characterized in 1994 as another negative regulator of T-cell activation.7 In 1996, Allison’s team8,9 reported that CTLA-4, similar to PD-1, serves as an inhibitory molecule that restricts the antitumor T-cell response. Their findings showed that the blockade of CTLA-4, like the blockade of PD-1, could also lead to enhanced antitumor immune responses with tumor rejection.8,10 Based on Allison’s research, blockade of the checkpoint molecule CTLA-4 with monoclonal antibodies8,9 has enabled the development of breakthrough therapies in oncology and ultimately led to the clinical development of ipilimumab (trade name Yervoy).11
However, it remains challenging to predict which patients are likely to respond to treatment with PD-1/PD-L1/CTLA-4 blockers. The discovery of biomarkers has assisted in patient stratification and management during immunotherapy treatment.12,13 The immune checkpoint proteins PD-1, PD-L1, and CTLA-4 are also proposed as predictive biomarkers for cancer immunotherapy in clinical practice. To guide clinical decisions, further validation of candidate predictive biomarkers is warranted.14–16
Most of the available data on CTLA-4 expression are limited to human T cells. However, the detection of the CTLA-4 protein in various cells other than T lymphocytes suggests its involvement not only in the established classical mechanism regulating T lymphocyte activity but also in the modulation of functions in other immunocompetent cells.17,18 The expansion of the existing ideas regarding the role of CTLA-4 may lead to the development of new approaches for improving diagnostic accuracy and suggest that CTLA-4 has more extensive effects on immune regulation.
The aim of our study was to analyze the phenotype and distribution pattern of predictive biomarkers in human melanoma. To achieve this, we performed immunophenotyping of CTLA4/PD1/PD-L1-positive cells in melanoma using double and triple immunofluorescent immunolabeling.19–21
Materials and methods
Patients
Melanoma tissue samples were obtained from 21 patients (five males and 16 females) with an age range of 30–82 years. All patients were in stage IV according to available clinical information. None of the patients had undergone immunotherapy with anti-PD-L1/PD-1 antibodies. A histopathological review was conducted for all patients, utilizing hematoxylin–eosin-stained slides to assess well-preserved tumor tissue and select the most representative tissue block. The selected specimens were subsequently screened by 3 pathologists in the absence of clinical information. Only patients with unanimous agreement on the histological diagnosis of malignant melanoma were included. The samples were obtained from the Institute for Hematopathology, Hamburg, Germany. These samples were redundant clinical specimens that had been de-identified and unlinked from patient information. Human tonsils were used as controls.
Ethics
This study was conducted in accordance with the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” and approved by the Institutional Review Board of the Institute for Hematopathology, Hamburg, Germany (F-2021-02; 22 January 2021). All the subjects provided informed consent before their inclusion. The samples were redundant clinical specimens that had been de-identified and unlinked from patient information.
Tissue probe staining
During routine histopathological procedures, remaining melanoma and control tonsil tissue probes were fixed in buffered 4% formaldehyde and embedded in paraffin. PBS was used for all washings and dilutions. Paraffin tissue sections (2 µm thick) were deparaffinized with xylene and rehydrated with graded ethanols. For pathohistological analysis, tissue sections were stained with H&E, covered with a permanent mounting medium, and observed using an upright microscope (Zeiss Axio Imager. Z1). The microscope was configured for transmitted-light brightfield microscopy and equipped with a Zeiss Axio Cam HRc microscope camera and a color CCD.
Immunohistochemistry
For immunohistochemical assays, deparaffinized sections were subjected to antigen retrieval by heating in a steamer with 10 mM sodium citrate buffer (pH 6.0) at 95°C for 30 min. We previously reported that endogenous Fc receptors in routinely fixed cells and tissue probes lose their ability to bind Fc fragments of antibodies; therefore, blocking endogenous Fc receptors prior to incubation with primary antibodies was omitted. After antigen retrieval, the sections were immunoreacted with primary antibodies listed in Table 1.
Primary antibodies used in this study
| Antibodies/Clone | Host | Source |
|---|---|---|
| Melan A (A103) | mouse monoclonal Ab | Ventana/Roche, Germany |
| SOX10 (SP267) | rabbit monoclonal Ab | Cell Marque, USA |
| CD1a (EP362R) | rabbit monoclonal Ab | Cell Marque, USA |
| CD3 (PS1) | mouse monoclonal Ab | AbCam, United Kingdom |
| CD3 (2GV6) | rabbit monoclonal Ab | Ventana/Roche, Germany |
| CD8 (SP57) | rabbit monoclonal Ab | Ventana/Roche, Germany |
| CD68 (KP-1) | mouse monoclonal Ab | Ventana/Roche, Germany |
| CD163 (MRQ-26) | mouse monoclonal Ab | Cell Marque, USA |
| CTLA-4 (UMAB249) | mouse monoclonal Ab | Origene Technologies |
| CTLA-4 (AA 57-86) | rabbit polyclonal Ab | antikoerper-online, Germany |
| CTLA-4 (SP355) | rabbit monoclonal Ab | AbCam, United Kingdom |
| CTLA-4 (CAL49) | rabbit monoclonal Ab | AbCam, United Kingdom |
| PD-1 (NAT105) | mouse monoclonal Ab | Cell Marque, USA |
| PD-L1 (ZR3) | rabbit monoclonal Ab | Zeta Corporation, USA |
Immunohistochemical visualization of the bound primary antibodies was performed either with a Ventana Slide Stainer or manually according to the standard protocol.19,20 For manually performed immunostaining, primary antibodies were applied at concentrations ranging from 1 to 5 µg/ml. PBS was used for all washings and dilutions. The bound primary antibodies were visualized using secondary antibodies (purchased from Dianova, Hamburg, Germany, and Molecular Probes, Darmstadt, Germany) conjugated with Cy3, Alexa Fluor-488 or Alexa Fluor-647. For the visualization of the primary anti-CTLA-4 antibodies, tyramide signal amplification (TSA) was used.20 A list of the secondary antibodies and other reagents used in this study is presented in Table 2.
Secondary antibodies and other reagents
| Antibodies and other reagents | Source | Dilution | Label |
|---|---|---|---|
| Goat anti-mouse IgG Ab (#A21236) | Invitrogen, Darmstadt, Germany | 1/100 | Alexa Fluor 647 |
| Goat anti-rabbit IgG Ab (#A21245): | Invitrogen, Darmstadt, Germany | 1/100 | Alexa Fluor 647 |
| Goat anti-mouse IgG Ab (#115-165-166) | DIANOVA, Hamburg, Germany | 1/200 | Cy3 |
| Goat anti-rabbit IgG Ab (#A-11034) | DIANOVA, Hamburg, Germany | 1/200 | Cy3 |
| Goat anti-mouse IgG A (#A-11029 | Invitrogen, Darmstadt, Germany | 1/200 | Alexa Fluor 488 |
| Goat anti-rabbit IgG Ab (#A-11034) | Invitrogen, Darmstadt, Germany | 1/200 | Alexa Fluor 488 |
| AmpliStain™ anti-Mouse 1-Step HRP (#AS-M1-HRP) | SDT GmbH, Baesweiler, Germany | ready-to-use | HRP |
| AmpliStain™ anti-Rabbit 1-Step HRP (#AS-R1-HRP) | SDT GmbH, Baesweiler, Germany | ready-to-use | HRP |
| 4′,6-diamidino-2-phenylindole (DAPI, #D9542-5MG) | Sigma, Hamburg, Germany | 5 µg/ml | w/o |
| VECTASHIELD® Mounting Medium (#H-1000) | Vector Laboratories, Burlingame, CA, USA | ready-to-use | w/o |
| CC2 solution (#950-223) | Ventana | ready-to-use | w/o |
| TSA Plus Fluorescein (#NEL741E001K) | PerkinElmer, Rodgau Germany | 1/100 | Fluorescein |
| TSA Plus Cyanine 3 (#NEL744E001KT) | PerkinElmer, Rodgau Germany | 1/100 | Cyanine 3 |
| TSA Plus Cyanine 5 (#NEL745E001KT) | PerkinElmer, Rodgau Germany | 1/100 | Cyanine 5 |
For simultaneous detection of antigens from the same host species, TSA with subsequent heat elution after each immunostaining step was performed.22 The bound primary/secondary antibody complex from the preceding immunolabeling step was eluted with citrate/acetate-based buffer (pH 6.0) containing 0.3% SDS (also available from VENTANA as CC2 solution, cat # 950-223). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, 5 µg/ml in PBS) for 15 s, and the sections were mounted using VectaShield (Vector Laboratories, Burlingame, USA).
The immunostained tissue sections were observed on a Zeiss Axio Imager. Z1 was configured for fluorescence microscopy and equipped with a Zeiss Axio Cam HRm microscope camera and a monochrome CCD.
Controls
Control incubations included either the omission of primary antibodies or substitution of primary antibodies with the same IgG species (Dianova, Hamburg, Germany) at the same final concentration as the primary antibodies. The exclusion of either the primary or the secondary antibody from the immunohistochemical reaction and substitution of primary antibodies with the corresponding IgG at the same final concentration resulted in a lack of immunostaining. The TSA step alone did not contribute to any specific immunostaining that might have influenced the analysis. Moreover, the specific and selective staining of different cells with primary antibodies from the same species using the same preparation method is by itself sufficient control of the specificity of immunostaining.
Results
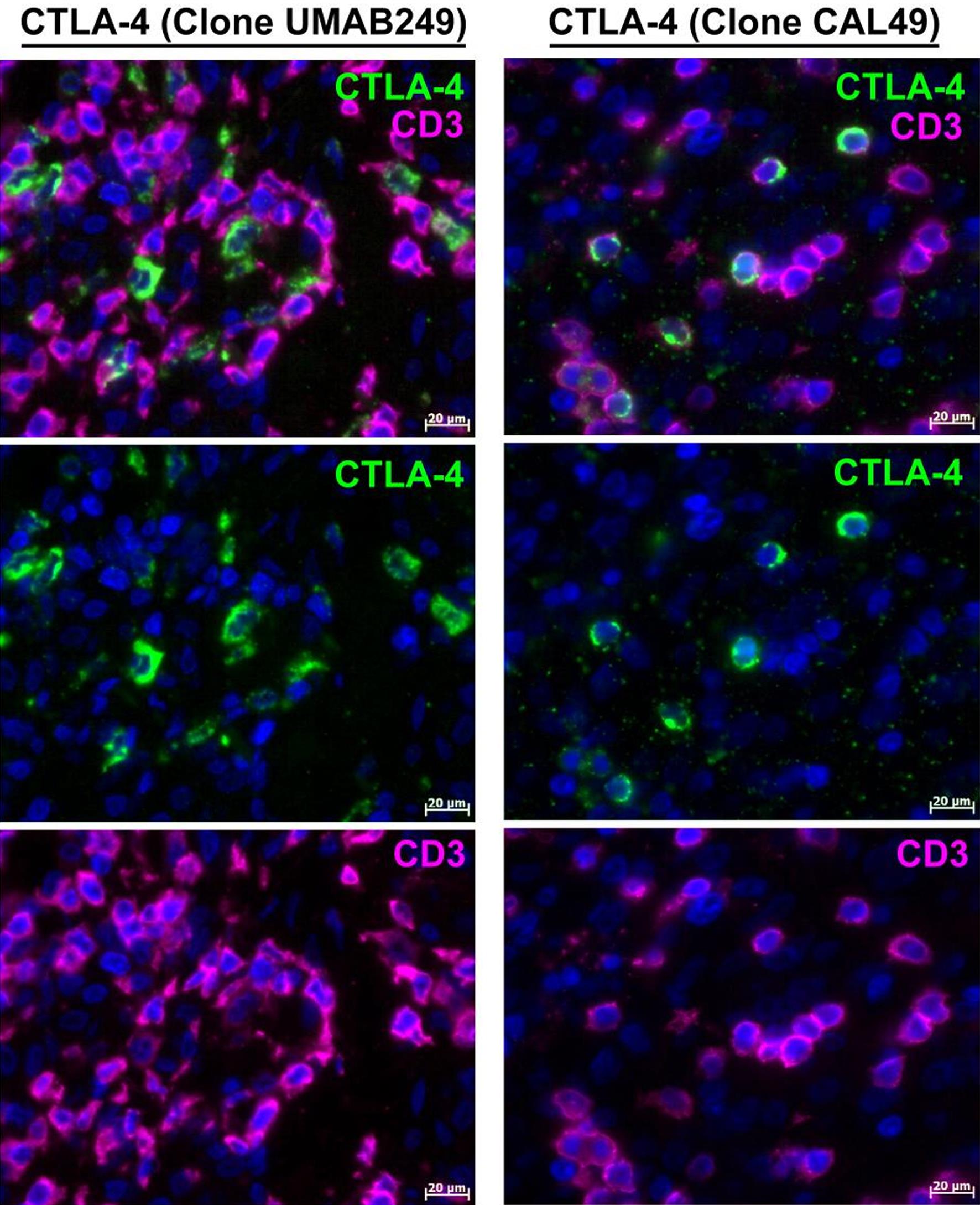
As CTLA-4 was originally identified as a T-lymphocyte antigen,23 we initially focused on its potential expression on T-lymphocytes. Antibodies against CD3 and CD8 as markers for T lymphocytes were used. For the immunolabeling of CTLA-4, four different anti-CTLA-4 antibodies against the clones UMAB249, AA 57-86, SP355, and CAL49 were used (Table 1).
With respect to the melanoma probes, double immunolabeling of T lymphocytes for CTLA-4 using anti-CTLA-4 antibodies against the CAL49 clone revealed the presence of the CTLA-4 protein on CD3-positive lymphocytes. In contrast, surprisingly, immunolabeling of T lymphocytes for CTLA-4 using the anti-CTLA-4 antibody of the UMAB249 clone revealed a lack of CTLA-4 protein in CD3-positive lymphocytes (Fig. 1). A lack of CTLA-4 expression on CD3-positive lymphocytes was also observed with the anti-CTLA-4 antibodies of the AA 57-86 and SP355 clones (data not shown). No significant individual differences in immunohistochemical labeling were observed among the patients.
The scale bar is 20 µm.
The lack of CTLA-4 immunolabeling (UMAB249, AA 57-86, and SP355 clones) on CD3-positive lymphocytes was observed in the control probes of the human tonsils in this study. This finding aligned with our earlier study on CTLA-4-positive cells in human tonsils.17
In the control probes of human tonsils, as reported in our earlier study,17 the absence of CTLA-4 protein on CD3-positive lymphocytes paralleled the findings in melanoma probes. This effect was particularly noticeable when using anti-CTLA-4 antibodies of the UMAB249 clone. Meanwhile, combining the CTLA-4 antibody of the CAL49 clone with CD3 or CD8 through immunofluorescence revealed that CTLA-4-positive lymphocytes in tonsils were typically positive for CD3 but only very rarely for CD8.
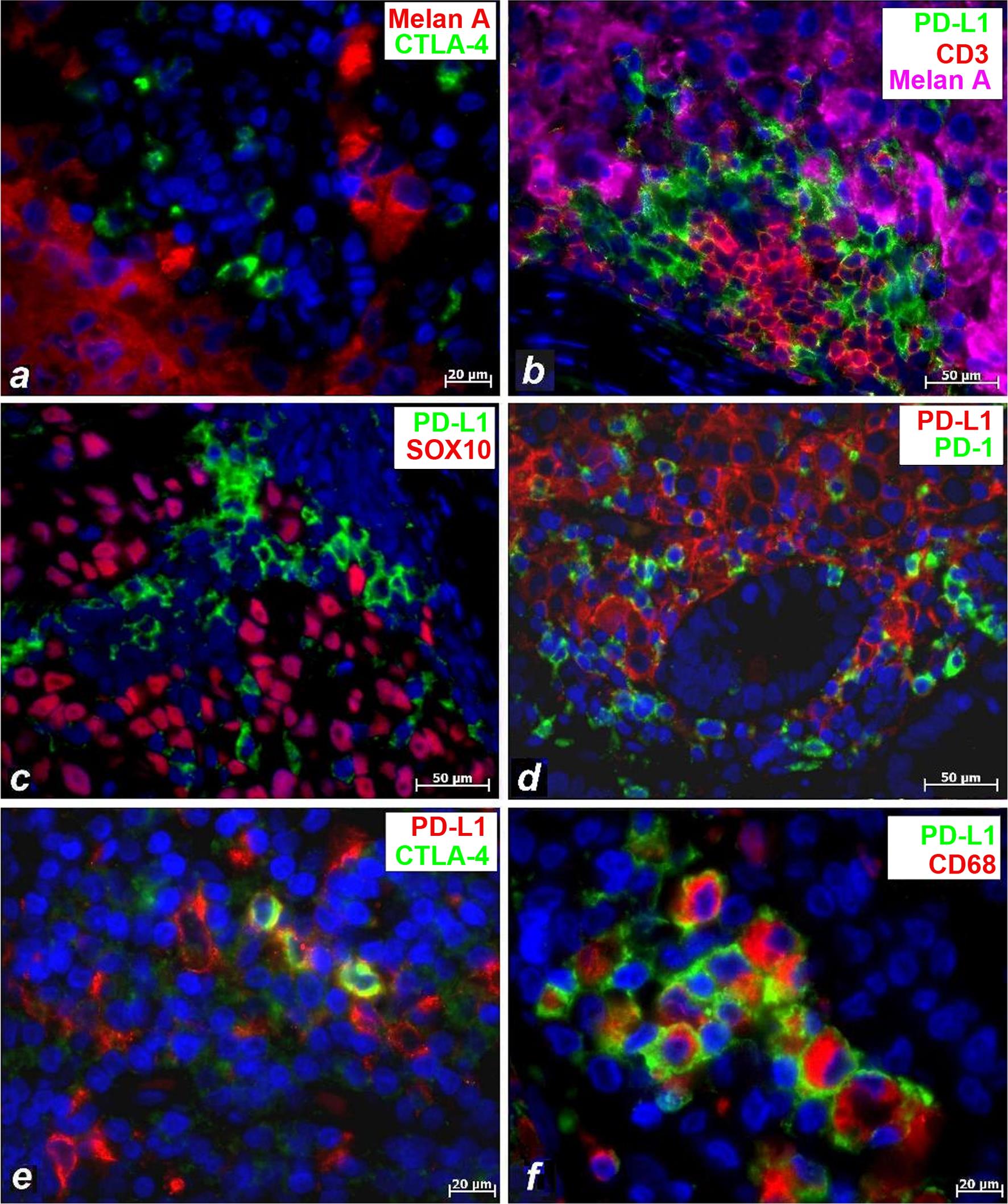
All further immunophenotyping of CTLA-4-positive cells in melanoma was performed using the anti-CTLA-4 antibody of the UMAB249 clone in combination with markers of melanocytes, macrophages, and dendritic cells. Malignant cells in melanoma were detected using two markers of melanocytes: Melan A (Fig. 2a and b) and SOX10 (Fig. 2c). It is notable that melanocytes were not found to express CTLA-4 (Fig. 2a). Double and triple immunolabeling of melanoma revealed that melanocytes immunolabeled with the Melan-A antibody (Fig. 2b) or with the SOX10 antibody (Fig. 2c) did not express PD-L1. PD-L1 was also absent in cells marked with CD3 (a marker of T cells) (Fig. 2b) or with PD-1 (Fig. 2d).
(a) Melanocytes do not express CTLA-4. (b) Immunofluorescent triple staining revealing expression of CD3, PD-L1 and Melan Aat different locations. (c) Immunofluorescent double staining revealing expression of SOX10 and PD-L1 at different poles. (d) Immunofluorescent double staining revealing expression of PD-1 and PD-L1 at different poles. (e) Immunofluorescent double staining revealing intracytoplasmic localization of CTLA-4 in some PD-L1-positive cells. (f) Immunofluorescent double staining revealing intracytoplasmic localization of CD68 in some PD-L1-positive cells. The scale bars are 50 µm for (b), (c), and (d) and 20 µm for the remaining images.
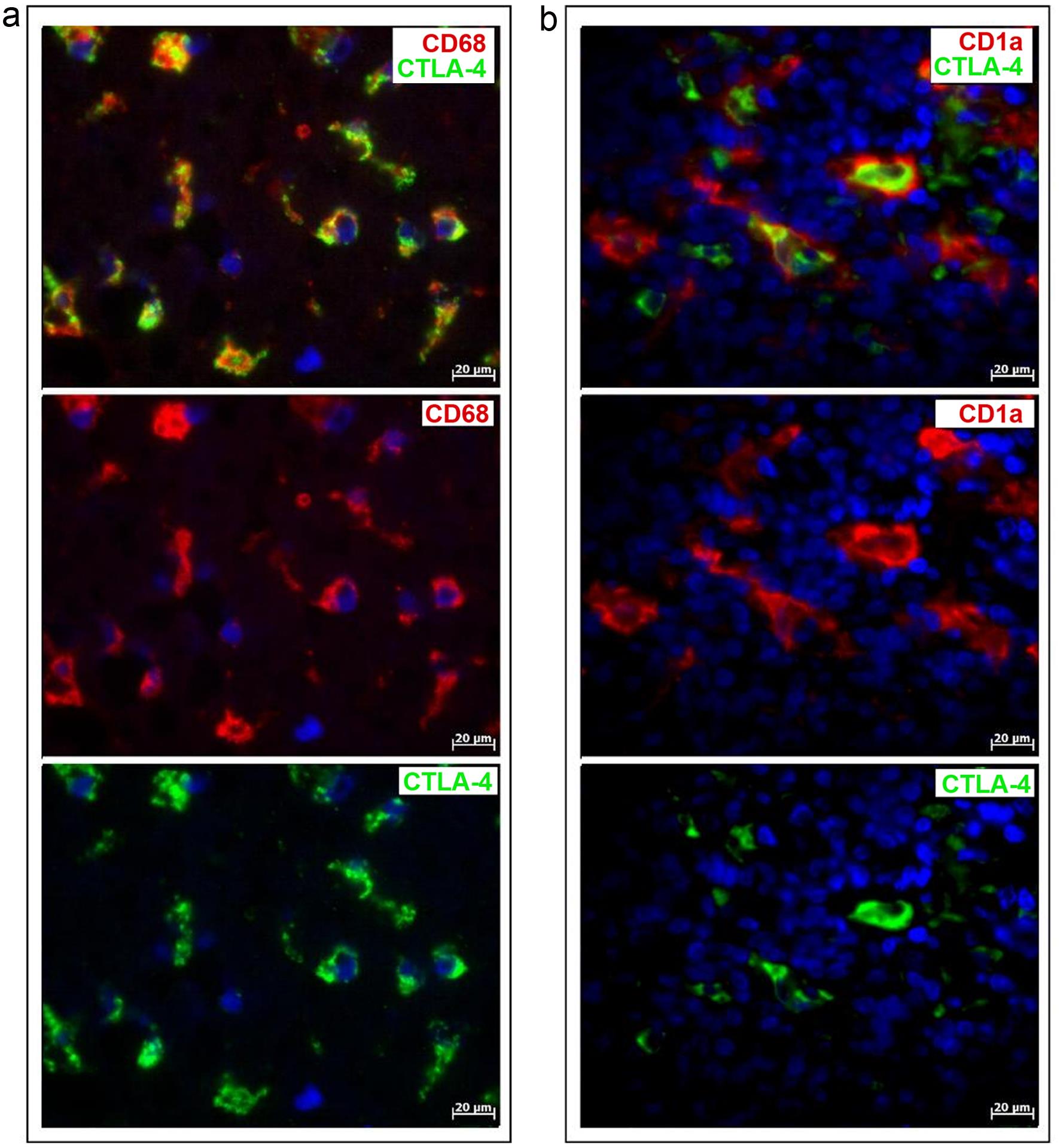
Our study demonstrated the coexpression of CTLA-4 (Fig. 2e) and CD68 (Fig. 2f) or CD163 (not shown) in PD-L1-positive cells, supporting the conclusion that CTLA-4 is expressed in macrophages in melanoma. This finding was further supported by double immunolabeling for CTLA-4 and CD68 (Fig. 3a). Notably, CTLA-4 was found within the cytoplasm (Figs. 2e and 3a), similar to CD68 (Figs. 2f and 3a), while PD-L1 was found on the surface of these cells (Fig. 2e and f).
(a) Immunofluorescent double staining of CD68 and CTLA-4 in the tumor microenvironment. (b) Immunofluorescent double staining of CD1a and CTLA-4 in the tumor microenvironment. The scale bar is 20 µm.
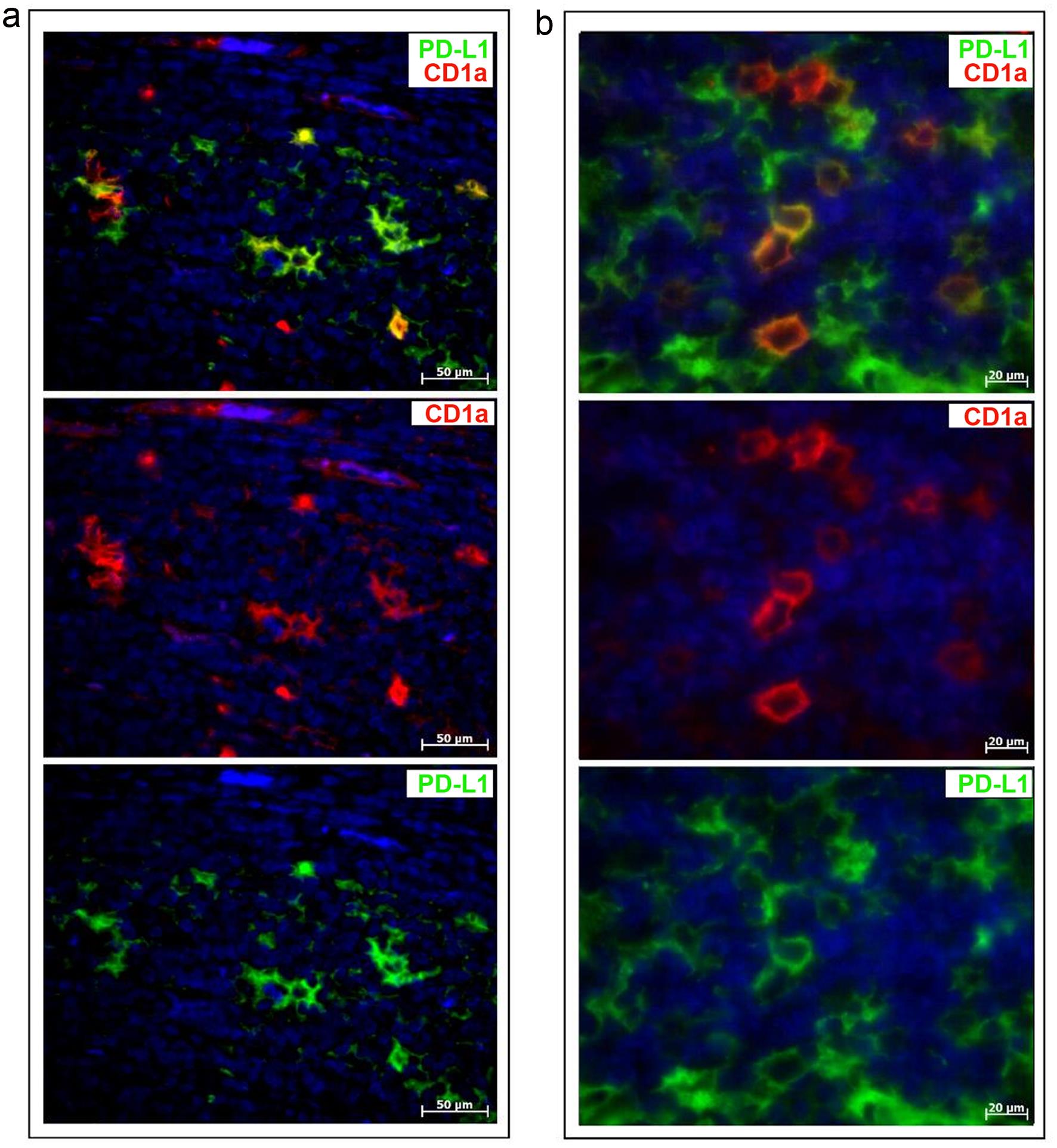
CTLA-4, which has a specific intracytoplasmic localization, was also found in dendritic cells labeled with the CD1a antibody (Fig. 3b). Like macrophages, dendritic cells were observed to coexpress PD-L1 (Fig. 4a and b). These findings led us to conclude that the immune checkpoint proteins PD-L1 and CTLA-4 are expressed in antigen-presenting cells, i.e., dendritic cells (Fig. 4a and b) and macrophages (Figs. 2e and 3a), but not in melanocytes (Fig. 2a–c), in melanoma. Moreover, another immune checkpoint protein, PD-1, was found to be localized exclusively in T lymphocytes (data not shown).
Discussion
Given the complexity of the tumor microenvironment and the dynamic interaction between tumors and immune cells, the PD-1/PD-L1/CTLA-4 regulatory pathways in tumors must be understood in greater depth; this makes it necessary to investigate predictive biomarkers.24
In malignant cells of various cancers, PD-L1 expression is graded from 0% to 50%.25 However, our study showed that malignant cells in melanoma lacked PD-L1 expression; instead, this immune checkpoint protein was found only in antigen-presenting cells, i.e., macrophages (Figs. 2f, 3a) and dendritic cells (Figs. 3b, 4a, b). This means that PD-L1 expression by tumor cells cannot serve as an absolute biomarker for predicting clinical response to checkpoint blockades in immunotherapy. This finding is in line with reports that patients for whom malignant cells in tumors lack PD-L1 expression also respond positively to PD-L1 checkpoint blockade therapies.26,27 Correspondingly, patients with overexpressed PD-L1 in the tumor microenvironment exhibit improved clinical outcomes after anti-PD-1/PD-L1-directed therapy.28,29 Therefore, PD-L1 expression in tumor tissue can be regarded as a more valuable biomarker than PD-1 expression in guiding clinical decisions.
The specific mechanism by which CTLA-4 regulates the immune response is complex and not fully understood.30 The intracellular localization of CTLA-4 in antigen-presenting cells in melanoma was consistent with reports that a key feature of CTLA-4 is its rapid and constitutive endocytosis from the plasma membrane, resulting in approximately 90% of CTLA-4 being intracellular.31 Initially identified by Pierre Golstein and colleagues as a T-lymphocyte antigen, CTL-4 was later described as a T-cell surface receptor,7 which, according to Allison’s concept,8,9 was later adopted as a target in melanoma immunotherapy. However, we found that T lymphocytes from human melanoma (Fig. 1), as well as from tonsils, lacked CTLA-4 expression when immunolabeled with anti-CTLA-4 antibodies against the UMAB249, AA 57-86, and SP355 clones.
Most of the available data on CTLA-4 expression are limited to human T cells. Unfortunately, CTLA-4 is often overlooked because it is present in cells other than lymphocytes. When using the anti-CTLA-4 antibody against UMAB249 clone, we detected CTLA-4 in melanoma cells in the tumor microenvironment, specifically in antigen-presenting cells, such as macrophages (Figs. 2f, 3a) and dendritic cells (Fig. 3b), but not in T lymphocytes. In contrast, immunolabeling of CTLA-4 with the antibody clone CAL49 vs the T lymphocyte markers CD3, CD4, or CD8 showed that the CTLA-4-positive T lymphocytes in human melanoma and tonsils were typically CD3+ and CD4+ but not CD8+.32
The detection of CTLA-4 protein in various cells other than T lymphocytes suggests the participation of this molecule not only in the established classical scheme for regulating T lymphocyte activity but also in modifying the function of other immunocompetent cells. Expanding the existing ideas regarding the role of CTLA-4 may lead to the development of new approaches for improving diagnostic accuracy and suggest that CTLA-4 has more extensive effects on immune regulation.
The differences in immunolabeling patterns with the anti-CTLA-4 antibodies from different clones can be explained by the generation of anti-CTLA-4 antibodies that recognize different epitopes of this protein. A decisive answer to the question of which cells constitute targets for ipilimumab (trade name Yervoy, developed by Bristol-Myers Squibb and used in the immunotherapy of melanoma) can be obtained via immunophenotyping. This involved the use of an anti-CTLA-4 antibody of the same clone as the monoclonal anti-CTLA-4 therapeutic antibody ipilimumab to identify CTLA-4-positive cells. This issue must further be assessed since it has direct therapeutic implications.
The detection of CTLA-4 proteins in cells other than T lymphocytes, using anti-CTLA-4 antibodies from different clones, is apparently due to the generation of anti-CTLA-4 antibodies that recognize different epitopes of this protein. This is an important finding that has implications for the effectiveness of different therapeutic anti-CTLA-4 antibodies.
Despite the enormous success and efficacy of anti-CTLA-4 therapy in patients with melanoma, subsequent clinical trials have shown that combination therapy targeting both CTLA-4 and PD-1 appears to be even more effective. This can be explained by the significant presence of T lymphocytes in the tumor microenvironment, as observed in melanoma probes (Fig. 2b, d); most CD3-positive cells were found to coexpress PD-1. Accordingly, better clinical outcomes with immune checkpoint therapy in melanoma can also be expected from combination therapy with the inclusion of PD-L1 as a target.33–35 Moreover, PD-L1 is strongly expressed in invaded macrophages in the melanoma tumor microenvironment (Fig. 2f) and dendritic cells (Fig. 4). These findings are in line with previous reports that a high level of PD-L1 expression on immune cells, rather than tumor cells, is a favorable prognostic factor.25
Taken together, our results indicate that CTLA-4 and PD-L1 are not expressed in malignant melanoma cells but are expressed in cells in the tumor microenvironment. Like PD-1 which is expressed on T lymphocytes, PD-L1 and CTLA-4 expressed in cells within the tumor microenvironment are valuable biomarkers for guiding clinical decisions. The detection of CTLA-4 proteins in various cells other than T lymphocytes suggests the participation of this molecule not only in the established classical scheme regulating T lymphocyte activity but also in modifying the function of other immunocompetent cells. The detection of CTLA-4 proteins in cells other than T lymphocytes using anti-CTLA-4 antibodies from different clones seems to be due to the generation of anti-CTLA-4 antibodies that recognize different epitopes of this protein. These findings have significant implications for the effectiveness of different therapeutic anti-CTLA-4 antibodies.
Abbreviations
- TSA:
tyramide signal amplification
- PD-1:
programmed death-1
Declarations
Acknowledgement
None.
Data sharing statement
All data and materials are available upon reasonable request; such requests should be submitted to I.B. (email:
Ethical statement
This study was approved by the Institutional Review Board of the Institute for Hematopathology, Hamburg, Germany (F-2021-02; 22 January 2021). This study was conducted in accordance with the principles of the World Medical Association’s Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” (as revised in 2013). All the subjects provided written informed consent before they were included in the study. The samples were redundant clinical specimens that had been de-identified and unlinked from patient information.
Funding
This work was supported by a departmental startup fund (HpH-2023-001) from the Institute of Hematopathology, Hamburg, Germany.
Conflict of interest
The authors declare no conflicts of interest.
Authors’ contributions
Conceptualization: I.B., A.K., and M.T.; Investigation: V.S. and D.A.; Writing—review and editing: I.B. and M.T.; Project administration: M.T. All the authors were actively involved with their work on this manuscript and have approved the final version of the manuscript.

 Author information
Author information