Introduction
Recently, articles on low-level laser therapy (LLLT) or photobiomodulation therapy have been increasingly found in the medical literature. It consists of a weak, mostly continuous wave laser irradiation of diseased tissue to increase physiological functions. Since it is difficult to determine “low-level,” the term photobiomodulation therapy or laser biomodulation therapy has been introduced. Relevant scientific publications using both terms can be found in search engines.
Natural light regulates metabolism, secretion, pigmentation, and day/night rhythm, and it is necessary for vision.1 Artificial laser light differs from natural light or light-emitting diodes by its monochromatic nature and especially by its coherence. Coherence means that all waves are in phase with each other in time, frequency, and spatial direction of propagation. Due to its coherence, laser light has a greater energy density and deeper tissue penetration, resulting in a stronger effect. Since the 1980s, diode lasers have been available on the market and used in human medicine for therapeutic applications.
To determine whether this innovative therapy can be useful in ophthalmology, laser irradiation using gallium-arsenide diodes with a wavelength of 780 nm, a power of 5–10 mW, and a frequency of 292 Hz has been employed to treat various eye diseases. LLLT has been shown to have positive therapeutic and protective effects in a variety of eye diseases, and it can even be used as a diagnostic tool, e.g., in glaucoma. It provides a noninvasive, nonpharmacological alternative or addition to traditional therapy, and it might help to reduce the total cost of ophthalmological treatments.
After many years of experience and accumulation of knowledge, and despite many remaining open questions, we have come to the conclusion that LLLT should be integrated into daily practice as a therapeutic option. Nevertheless, larger studies are necessary to confirm the promising experiences to date. This article gives a short overview of the effects of LLLT in common therapeutic applications in ophthalmology.
Application of LLLT
Laser light is applied by using a prototype laser device (Bimed, Munich, Germany), with its nonfocused continuous wave diode having a wavelength of 780 nm, a frequency of 292 Hz, and a peak power of 1–10 mW. We have previously found in the pig eye that to cross all layers of the eye, 10.3 mW is required.2
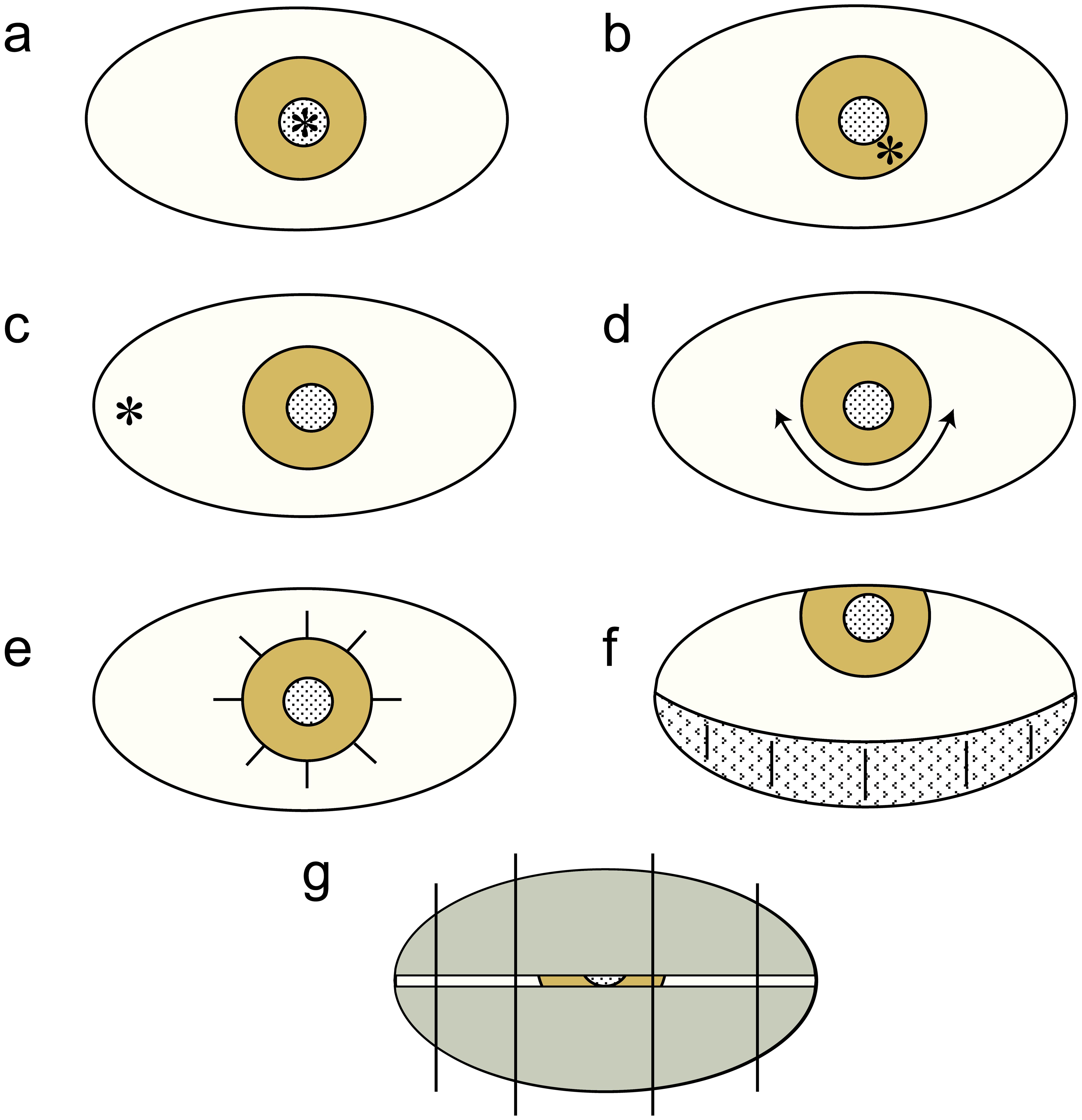
The pathological condition determines the duration and the type of treatment. Mainly the affected location is irradiated. The light can be applied transpupillary, transconjunctival, palpebral, or parabulbar. Figure 1 shows the different modes of application of laser irradiation to the eye. However, to avoid possible glare or afterimages, the irradiation is mostly transconjunctival/transscleral.
(a) Transpupillary; (b) transcorneal; (c) transconjunctival; (d,e) limbal; (f,g) palpebral-transcutaneous.
The duration of irradiation is usually 30 s per point area (dose: 300 mW/cm2), while the laser light is constantly moved to avoid local overdosage. It has been shown to be advantageous to first irradiate for three days, once per day; then, if necessary, irradiate once or twice a week until the desired result is achieved.
Neither the treating physician nor the participants in these investigator-initiated trials received any financial or other benefits. Considering the Declaration of Helsinki and after prior informed patient consent, the treatments were performed with LLLT.3
Treatment of amblyopia in adults
Visual acuity is not stable and is known to be easily “irreversibly” lost in various eye diseases, e.g., amblyopia. For this disease, treatment options are often limited or even nonexistent.
In one study of 178 adult patients, 231 eyes with amblyopia were treated with LLLT and examined for changes in visual acuity.4 Both ametropic eyes with amblyopia (110 eyes, 91%, p < 0.001) and eyes with strabismic amblyopia (121 eyes, 89%, p < 0.001) experienced visual acuity improvement in the best-corrected visus. Irradiation was performed in the temporal inferior quadrant, at maximum adduction, on average three times without occlusion (laser: 780 nm, 7.5 mW, 292 Hz, total dose: 675 mW/cm2). To confirm the objective change, visual evoked potentials were derived on 12 strabic amblyopic eyes according to criteria published on www.iscev.org . The amplitude improved by 1,207 nV (50%, p < 0.001), and the latency shortened on average by 7 ms (5.3%, p < 0.14).5 In patients with strabic amblyopia, the vision of the leading eye remained on the improved level, whereas the vision of the nonleading eye returned to the baseline value over time but could be improved again by repeated laser irradiation. Also, a reduction of the squint angle was observed. However, further studies are needed to confirm these findings.
Glaucoma
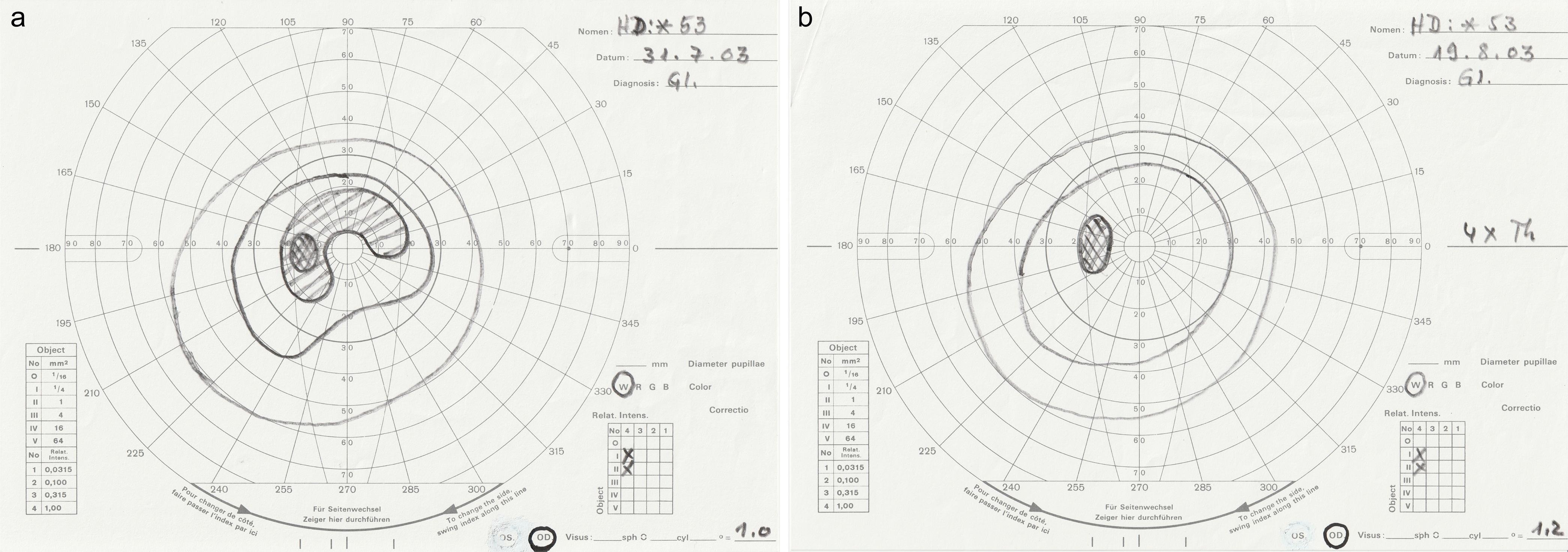
Glaucoma is a chronic progressive optic atrophy, a secondary mitochondriopathy, caused by several risk factors. Chronic ocular hypertension is considered the main risk, causing retinal damage, which manifests as visual field and visual acuity loss. The current drug treatment is insufficient to eliminate the disease. To reduce retinal damage and to normalize the visual field, 63 glaucomatous eyes of 38 patients (22 females, 16 males, average age: 58.4 years) were treated with LTTT as follows: First, to lower the intraocular pressure (IOP), the limbus was irradiated for 30 s (Fig. 1a), on average three times (675 mW/cm2); then the retinal damaged area was additionally irradiated (Fig. 1b) for 60 s, two times per week. On average, 7.5 treatments were performed per patient (4.5 W/cm2). Of 63 eyes with optic atrophy and defective visual fields, 32 (51%) had completely normalized visual fields after LTTT (Fig. 2), and 29 (46%) had more than 10 degrees of improvement. One patient dropped out of the study for personal reasons. His visual field results (n = 2) were excluded from analysis. Normalization of all visual fields was not attempted. The visual evoked potentials by criteria given at www.iscev.org were derived in 15 eyes. The amplitude increased by 877 nV, on average (13%, p < 0.001), and the latency decreased by 13.5 ms, (8%, p < 0.001). The visual acuity improved significantly (70.8% of eyes), and the IOP was decreased by 9.9 mm Hg, on average (39.7%, p < 0.001).
(a) Before treatment. (b) After irradiation four times in three weeks.
Weak laser light also is suitable for the reliable early diagnosis of ocular hypertension and thus eliminates possible diagnostic uncertainties. For this purpose, the effect of the laser irradiation (780 nm, 7.5 mW, 292 Hz, dose: 225 mW/cm2) can be assessed as follows:5 First, perform a diagnostic test for ocular hypertension using LLLT for applanation tonometry to determine the baseline IOP value. Then, perform laser irradiation in the limbal region (Fig. 1a) for 30 s (dose: 225 mW/cm2). After 30 min, perform the second applanation tonometry and subtract both IOP values. For example: IOP before irradiation (21 mm Hg) – IOP after irradiation (15 mm Hg) = IOP difference (6 mm Hg; 28.6%, indicating mild ocular hypertension). The test is repeated in the same way until the IOP value remains unchanged. The achieved chemical-energetic saturation does not allow any further IOP change by the weak laser irradiation. This final IOP value can be regarded as the individual physiological IOP value.3 Applying this test scheme, a better separation of healthy from diseased eyes can be achieved. At the same time, ocular hypertension can be treated by IOP lowering, as described above.4,6
Age-related macular degeneration (AMD)
AMD is a progressive disease of the macula that can lead to visual loss and blindness with age. Treatment attempts for the exudative stages are long-lasting, expensive, and with uncertain results. For the dry, intermediate stages of AMD, there is no satisfying therapy yet.
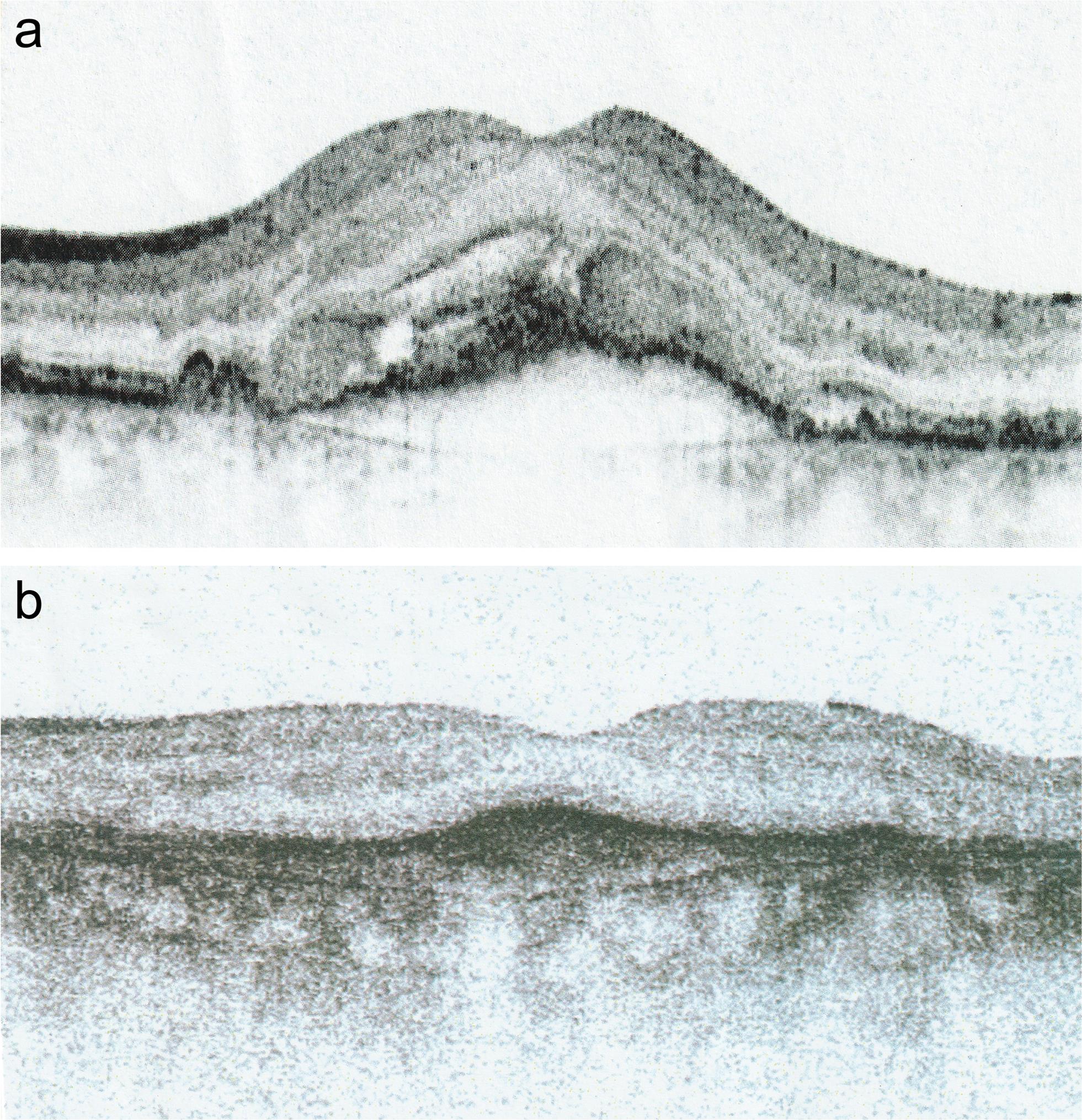
A study examined the effect of LLLT on 183 patients and 328 eyes with AMD in all stages.7 A total of 36 eyes had an exudative-hemorrhagic stage, while 182 eyes had mostly incipient cataracts in addition to AMD. Laser irradiation was performed in the inferior temporal quadrant, twice a week, for 40 s (laser: 780 nm, 7.5 mW, 292 Hz frequency, total dose: 900–1,200 mW/cm2). The eye was in maximal adduction during the treatment. The lower eyelid was kept pulled down. On average, treatment was performed 3–4 times. In both groups, with and without cataracts, visual acuity was improved in 95% and 97% of eyes, respectively (p < 0.00001). In the same way, retinal degeneration with high myopia and chorioretinitis centralis serosa was successfully treated without side effects.3 Acquired dyschromatopsia, metamorphopsia, and scotomas improved rapidly by LLLT as the edema and exudates were reabsorbed; after prolonged treatments, wet AMD became dry (Fig. 3). Drusen became scarcer, and the retina appeared more vital.7–9
Diabetic retinopathy
Besides AMD and glaucoma, diabetic retinopathy still frequently leads to blindness, representing a severe and widespread problem. Fifteen patients (30 eyes) with diabetic retinopathy at an early stage were treated with laser light (780 nm, 10 mW, 292 Hz), transconjunctivally, for 40 s, twice a week, on average six times (total dose: 2.4 W/cm2). The edema and exudates as well as bleedings were absorbed quickly, and visual acuity increased in all eyes.3 Since there are not always satisfying treatment options available,10 laser irradiation gives hope; however, further clinical studies are needed to confirm these findings.
Retinitis pigmentosa
In a case report, a patient showing typical signs of retinitis pigmentosa with optic atrophy was described to react positively to LLLT. The patient showed distinct improvement of both visual acuity and visual fields. After five years without treatment, both had returned to their initial findings; however, when LLLT was applied again, it achieved a similar positive effect.11
Eye inflammation
Regardless of their etiology, all types of ocular inflammation can be treated with LLLT alone or in combination with medication. With keratitis, the affected area and the limbal region are irradiated; while with uveitis, the iris is irradiated transcorneally. In the case of vitreous involvement, both transpupillary and external transconjunctival-scleral, or a combined irradiation can be used.
In 31 patients (33 eyes) with uveitis, irradiation was performed five times, for 30 s, daily, on average (dose: 300 mW/cm2). The effect was comparable to a treatment with cortisone, but without its side effects. Moreover, LLLT has been shown to be particularly effective in the treatment of neuritis retrobulbaris.12
Discussion
The effect of LLLT is complex and not yet fully understood. On a cellular level, laser light has a positive effect on the energy level of the cells. At a given moment, cells have a certain amount of internal metabolic energy in addition to their morphological structure.1 The energy is permanently consumed and synthesized in the mitochondria in the form of ATP molecules (adenosine triphosphate) by the conversion from glucose and with the consumption of oxygen. The ATP molecules are the main stores and suppliers of cellular energy. Their hydrolysis releases the valence energy required for physiological processes.13–15
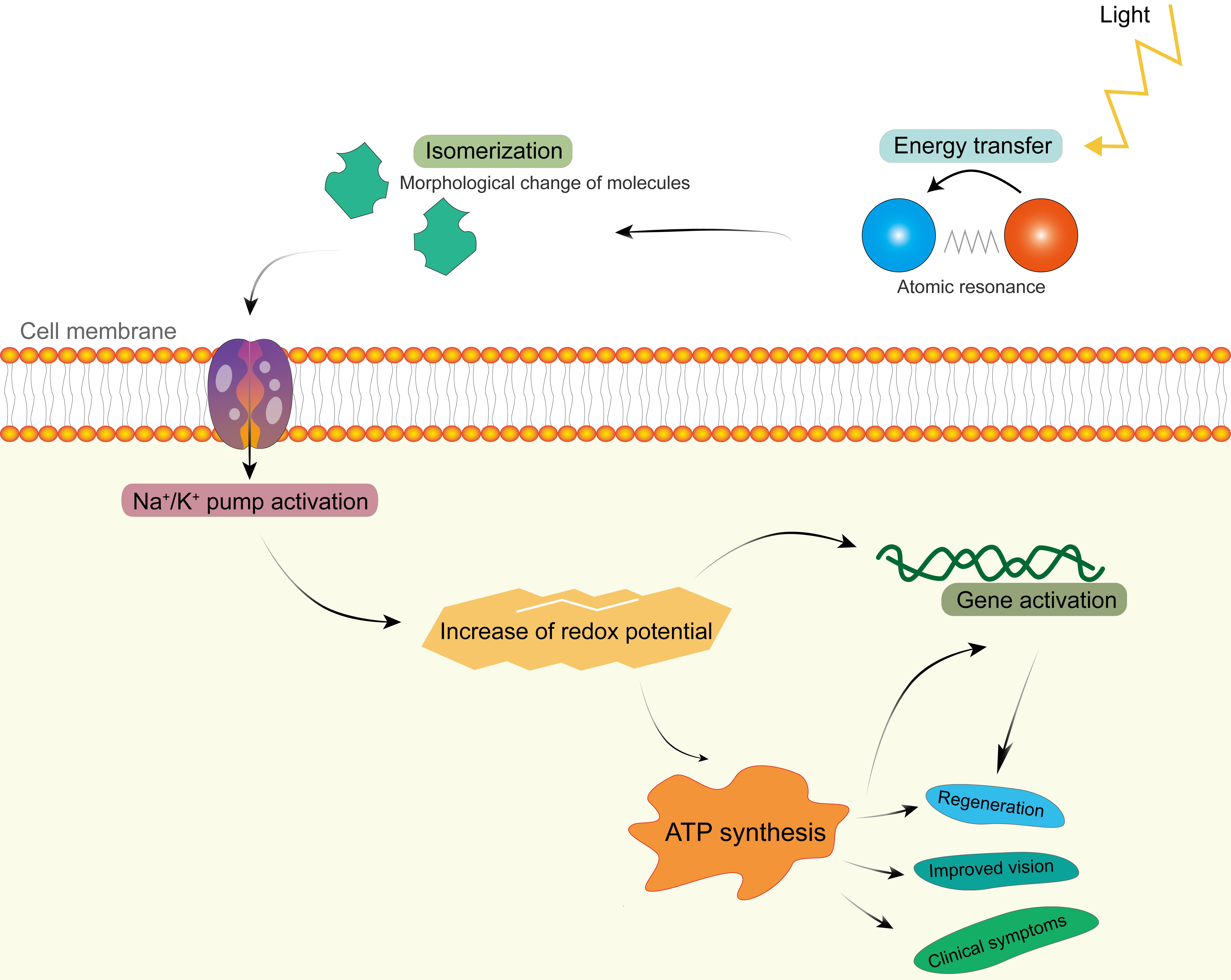
When the laser light hits matter, a purely photophysical process will occur first (Fig. 4), which is the basis of LLLT. The absorbed photon energy causes a morphological change on the acceptor molecule, its isomerization (e.g., rhodopsin changes its structure, color, and absorption maxima).16 Such a modification of the conformation takes place in picoseconds and also affects the molecules forming ion channels, e.g., for sodium and calcium, in the cell membrane. This leads to a change of the pH in the cytosol, subsequent activation of enzymatic processes, and an action potential, which is a change in the charge of the cell membrane. Normally, cells have a negative internal resting potential charge. The one of rods is -30 mV, which increases to -70 mV after the light stimulus, i.e., the cell becomes hyperpolarized.17 This hyperpolarization seems to be the most important change caused by LLLT. It increases the redox potential for the respiratory chain,3,14,17 cell metabolism, and secretion as well as improves the visual process. The potential generates itself by constant interactions on the elementary photon-electron level.3Fig. 4 illustrates the cascade from the physical energy impact of laser light to the effect on clinical signs.
LLLT has a substantial positive effect on various ophthalmological pathologies by causing hyperpolarization on a cellular level. It enhances physiological processes and can be considered a stimulus of regeneration and a regulator at cellular level, resulting in an effective therapeutic option to maintain vision, to revitalize tissue, and to recover lost physiological cell functions.
At 30 min after irradiation, it is possible to determine the changes in distance visual acuity, probably caused by a faster regeneration cycle of rhodopsin. Acquired dyschromatopsia improves, which indicates regeneration of cones. They regenerate first, then the rods.3 However, to improve visual acuity, a direct transpupillary irradiation of the affected macula is not necessary.
No single substance used in glaucoma monotherapy lowers the IOP as fast and strong as LLLT. After 10 min, the outflow of the aqueous humor increases sharply.18 LLLT seems to inhibit the sympathetic nervous system, while the parasympathetic nervous system is stimulated. In addition, the macrophages activated by laser irradiation phagocytose the cellular debris, and the aqueous humor drainage pathways are cleared and kept free.19,20 The diagnostic use of LLLT to determine the individual physiological IOP is helpful to steer the therapy in glaucoma patients. To determine this baseline value so easily has not been achieved by any other means.
LLLT leads to positive functional and morphological changes of the retina,3 possibly introduced by an increased cell metabolism. The edema and exudates rapidly retract, the hemorrhages are lysed by enzymes, and the cellular debris is phagocytosed by activated macrophages.19,21
After a light stimulus, new synapses form within seconds and function better for days or permanently.6,22 The formation of new synapses and the growth of dendrites and axons result in better intercellular connection and communication. In addition, gene activation increases. Generally, the retina, similar to the central nervous system, has only limited means to regenerate after damage due to the secretion of growth inhibitors by oligodendrocytes. However, as the retina has no oligodendrocytes, it is able to regenerate better. An increased blind spot might start to shrink already after three laser irradiations. Relative scotomas and impaired visual fields need 6–12 or even more therapy sessions, depending on their extent. The treatment should be continued until the visual field is normalized and the IOP remains stable.
The positive effects of LLLT on eye inflammation can be mediated in different ways. On the one hand, pain relief can occur by depolarization disruption, fibroserous exudates, and edema absorption. On the other hand, laser light activates microglia and macrophages as well as lowers the production of strongly proinflammatory cytokines such as tumor necrosis factor alpha, inducible nitric oxide synthase, cyclo-oxygenase 2, interleukin 6, and interleukin 8; whereas protective proteins are formed at a higher rate.12,20,23
Generally, the effect of LLLT over time depends on the initial pathological situation. The condition might remain at an improved level at least for months and up to three years. The eyes should be controlled every 3–6 months, and laser irradiation repeated, to prevent or invert regression.
Conclusions
LLLT has a physiological stimulative, regulative, regenerative, and anti-apoptotic effect. Ideally, it protects the eye from visual acuity loss and blindness. As an alternative or additional therapeutic option, it might help to reduce the total cost of therapy for ophthalmological conditions. Nevertheless, additional studies are necessary to confirm the promising experiences described to date.
Declarations
Acknowledgement
None.
Funding
None.
Conflict of interest
The author has no conflicts of interest to declare.

 Author information
Author information