Introduction
D-serine (D-ser) functions as a co-agonist of the glutamate-bound N-methyl-D-aspartate receptor (NMDAR), regulating neurotransmission and synaptic plasticity. Through this channel-type receptor, D-Ser modulates long-term potentiation, neuronal migration, and excitotoxicity.1–6 To potentiate NMDAR activity, D-Ser binds to the GluN1 subunit, in concert with GluN2-bound glutamate, opening the receptor and allowing the influx of sodium or calcium.1,4
D-Ser in the brain
D-Ser is primarily generated from levorotary serine (L-Ser) through a racemization reaction by serine racemase (SR), a pyridoxal 5′-phosphate-dependent enzyme, and degraded through oxidative deamination by the peroxisomal enzyme D-amino acid oxidase (DAAO), a flavine adenine dinucleotide-containing enzyme.1,7 SR also degrades D-Ser by α,β-elimination dehydration.8 In rodents and humans, SR is enriched in the forebrain while DAAO is substantially expressed in the hindbrain and spinal cord.9–11 Correspondingly, D-Ser is enriched in the forebrain and present in lesser amounts in the hindbrain and spinal cord in adults.11 Regulation of D-Ser by SR and DAAO shows regional differences in the brain because of the distributional difference between the two enzymes. In the rodent forebrain, knockout of SR reduces the D-Ser level by 90%, whereas loss of DAAO does not change the D-Ser level in this area.12 On the other hand, loss of DAAO increases the D-Ser level by more than 20 times in the hindbrain and spinal cord, while knockout of SR minimally changes the D-Ser level in these areas.12,13 Thus, SR determines the D-Ser level in the forebrain, and DAAO controls the level in the hindbrain and spinal cord.
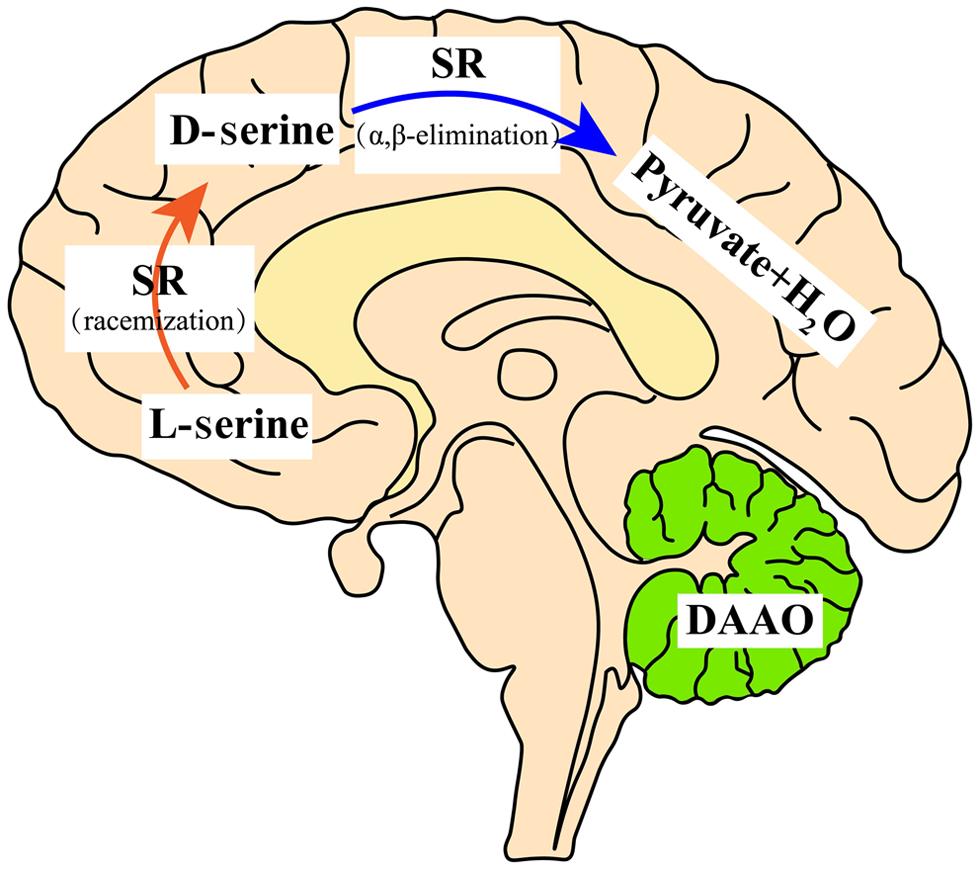
Tracing the cellular origin of D-Ser, SR is mostly confined to protoplasmic astrocytes according to the early studies; however, recent data indicate that SR is mostly localized to glutamatergic neurons, although reactive astrocytes and microglia express some SR.6,14–19 Regarding the cellular distribution, DAAO is preferentially confined to astroglia in the hindbrain.20 The distribution of D-Ser in the central nervous system is depicted in Figure 1.
In the forebrain, D-serine is abundant. SR synthesizes D-serine via racemization, while D-serine is degraded via an α,β-elimination reaction because of DAAO deficiency in the forebrain. In the adult cerebellum, D-serine is less abundant due to DAAO degradation. DAAO, D-amino acid oxidase; SR, serine racemase.
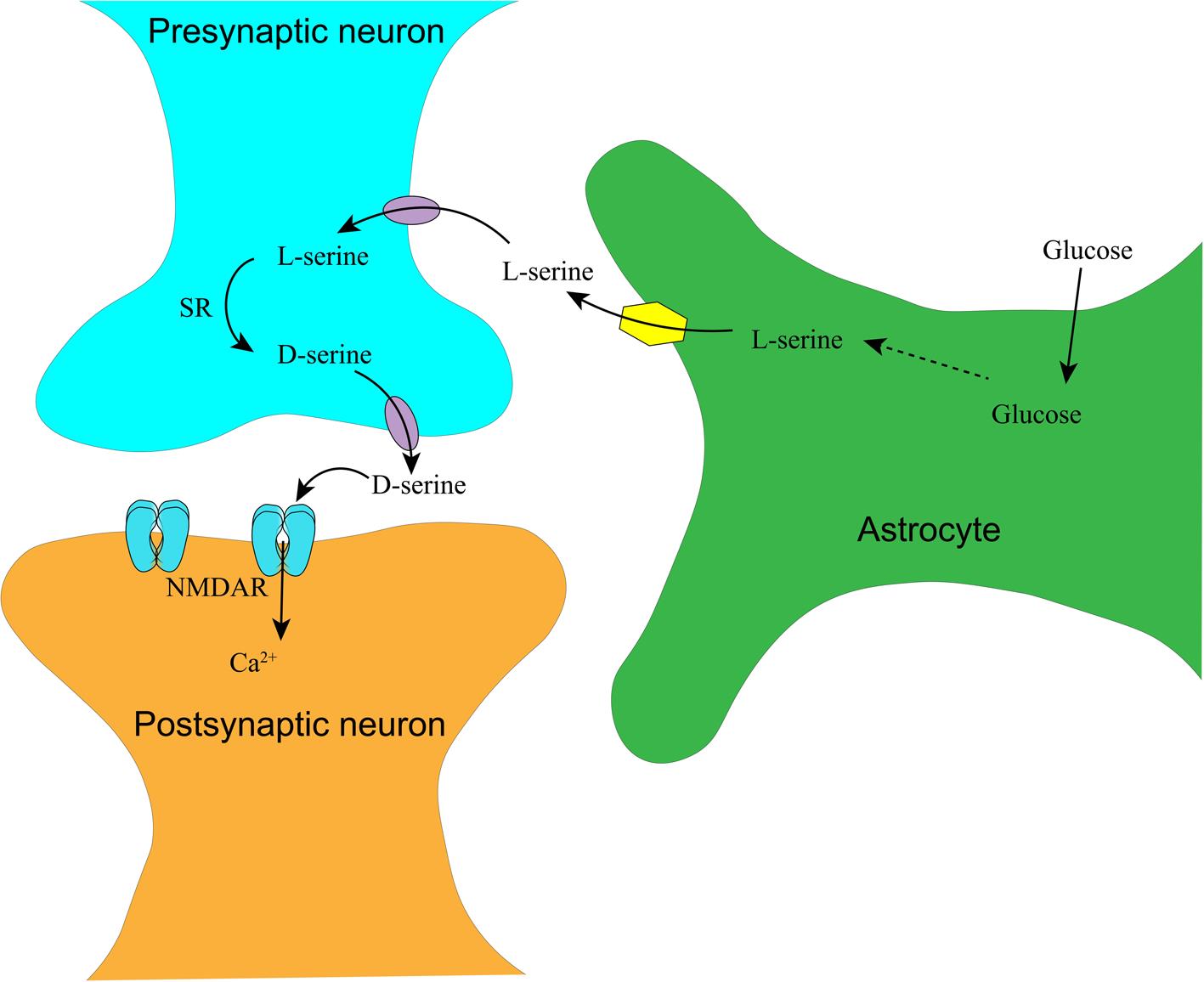
Appropriate NMDAR activity is also dependent on serine transport between astrocytes and neurons. To explain this transport, Herman Wolosker has proposed a delicate model dissecting serine transport in the brain: astrocytes take up glucose from the peripheral blood and metabolize it to L-Ser. L-Ser is then transported into presynaptic neurons, where it is converted to D-Ser by SR. D-Ser is transported out and acts on the NMDAR on the postsynaptic membrane.21 The synthesis and transportation of D-Ser in the central nervous system are depicted in Figure 2.
In the tripartite synapse consisting of pre-/post-synaptic neurons and enveloping astrocytes, glucose is taken up into astrocytes via glucose transporter 1 and synthesized into L-serine. L-serine is transported into the presynaptic neuron via a neural amino acid transporter, where L-serine is converted into D-serine by SR. Released D-serine and glutamate from the presynaptic neuron act on NMDAR on the postsynapse, triggering calcium or sodium influx. NMDAR, N-methyl-D-aspartate receptor; SR, serine racemase.
Inappropriate activity of NMDAR is implicated in neuropsychiatric disorders, including Alzheimer’s disease and schizophrenia.22–24 Overactivation of NMDAR leads to excitotoxicity, a shared mechanism in neurodegenerative disorders and stroke; whereas hypofunction of NMDAR is associated with schizophrenia.23–25 The use of high-affinity and strong competitive antagonists of NMDAR targeting the GluN2 subunit leads to gross neurological impairment and behavioral toxicity, whereas the side effects of uncompetitive antagonists are moderate.26 Thus, regulation of D-Ser availability seems much more tolerable. Blockade of D-Ser on the glycine-binding site of NMDAR or depletion of D-Ser by chemicals or genetic manipulation effectively attenuates NMDAR activity and greatly reduces excitotoxicity.4,6,27,28 For example, knockout of SR substantially protects against cerebral ischemia in rodents and, in doing so, greatly reduces injury in the NMDA/amyloid peptide-injected brain in rodents.28,29 Similarly, a mutation in DAAO leading to excessive production of D-Ser is associated with motor neuron degeneration in amyotrophic lateral sclerosis.30
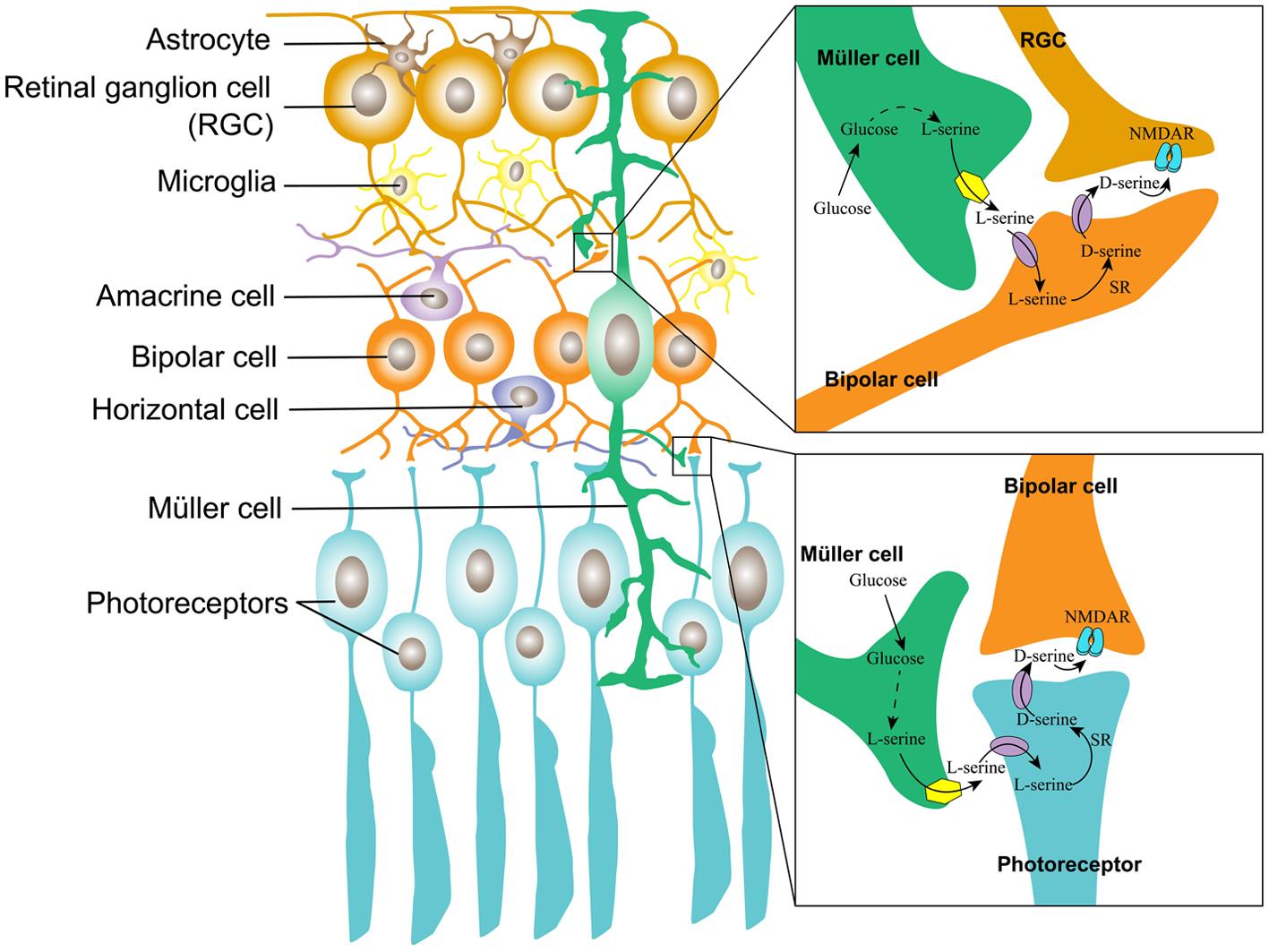
The Müller cell is the major type of glial cell in the retina and the outer retina; it provides trophic and anti-oxidative support for photoreceptors as well as maintains the inner blood-retinal barrier. In the inner retina, Müller cells regulate synaptic activity by uptaking glutamate.31 By mimicking the serine shuttle between astrocytes and neurons in the brain, we propose a similar model in the retina, which is depicted in Figure 3.
The Müller cell constitutes a tripartite synapse in the inner and outer retina. The Müller cell takes up glucose from the blood and metabolizes glucose to L-serine. In the inner retina, L-serine is transported into a bipolar cell, where it is converted into D-serine, which is transported out and acts upon NMDAR on the RGC; in the outer retina, L-serine is transported into the photoreceptor, where it is converted by SR into D-serine, which is released to the synaptic cleft, acting upon NMDAR on the bipolar cells. NMDAR, N-methyl-D-aspartate receptor; RGC, retinal ganglion cell.
The physiological roles of D-Ser in the retina
In the retina, D-Ser and SR are expressed in astroglia, Müller glia, retinal ganglion cells, and retinal pigment epithelial (RPE) cells.32–34 By comparison, SR in the retina is expressed at approximately the same level as that in the cortex.32 In detail, SR expression is greater in retinal neurons than in RPE cells.35 Early studies indicate that DAAO activity is at a relatively higher level in the inner plexiform layer of the rodent retina and also in the peroxisome of RPE cells and Müller glia in the amphibian retina.36,37 Our recent study also indicates that DAAO is mostly expressed in the RGCs, outer plexiform layer, and RPE cells in the rodent retina.38 In addition, knockout of SR decreases the D-Ser level in the mature retina by 90% relative to that of wild-type mice; while in the DAAO mutant line, the D-Ser level in the retina increased by only two-fold compared with that of wild-type mice.39 These analyses suggest that in the rodent retina, similar to the brain, D-Ser is principally synthesized by SR; while DAAO is not the only enzyme to degrade D-Ser in the retina, different from the dominant effect of DAAO on D-Ser degradation in the hindbrain and spinal cord.12
The function of D-Ser in the retina is much less clear than in the brain. In the mammalian retina, functional NMDAR is mostly localized to RGCs, the inner nuclear layer, and photoreceptors.32,40 In the inner retina, D-Ser inhibits non-NMDA glutamate receptors, recruits NMDAR, and mediates light-induced RGC current.36–41 Deletion of SR removes the NMDA component of the light-induced synaptic response but does not affect visual function.42 Meanwhile, in DAAO-knockout mice, exogenous application of D-Ser potentiates the NMDAR current in RGCs, suggesting that DAAO mediates subsaturation of the NMDAR glycine site in the retina.36 In contrast, the functional roles of D-Ser in the outer retina are less clarified. NMDAR hypo- and hyperfunction in the retina alter the components of the flash electroretinogram. The use of this technique demonstrated that deletion of SR significantly delayed the a-wave and reduced the b-wave amplitude, and the effects were more prominent in male Srr-knockout mice; meanwhile, the application of exogenous D-Ser through overactivation of NMDAR similarly alters the retinal field potential, but these changes are not observed in female wild-type mice.43 These differences suggest that the glycine-binding site of NMDAR is less saturated in the retina of male mice.
D-Ser may play a role in the early development of the retina. The neonatal retina consists of a double-cell layer, which is composed of an inner and an outer neuroblastic layer, that is separated by the inner plexiform layer, and it grows into a triple-cell layer at two weeks after birth, with a structure similar to that of the adult retina.44 D-Ser in the retina peaks in neonatal mice, followed by a dramatic decrease as the mice grow into adults, suggesting that D-Ser may play a role in the early development of the retina.33,39
The pathogenic roles of D-Ser in diabetic retinopathy (DR) and glaucoma
DR affects one-third of diabetic patients when the duration of diabetes lasts over a decade.45 The characteristics of DR include the loss of retinal vascular endothelial cells and pericytes as well as microaneurysm formation in the early stage and preretinal/vitreous hemorrhage, retinal neovascularization, and retinal detachment at the later stage of DR.46 It has taken a long time to recognize that DR is also a neurodegenerative disorder, and the neurodegeneration in the retina antedates overt retinal microvascular pathologies in diabetic animals and humans.47–50 The pathologic cascade of DR possibly begins with a retinal neurovascular uncoupling, retinal neurodegeneration, and reactive gliosis, followed by the ensuing disruption of the blood-retinal barrier and more severe retinal microvascular abnormalities.51,52 Conversely, disruption of the blood-retinal barrier causes infiltration of blood-born leukocytes and activation of residential microglia, which can compromise neuronal function and viability via cytokines and oxidative stress.53,54 Thus, neurodegeneration and injuries to the retinal microvessels mutually aggravate during DR progression, culminating in avalanche-like abnormalities at the end stage of DR.
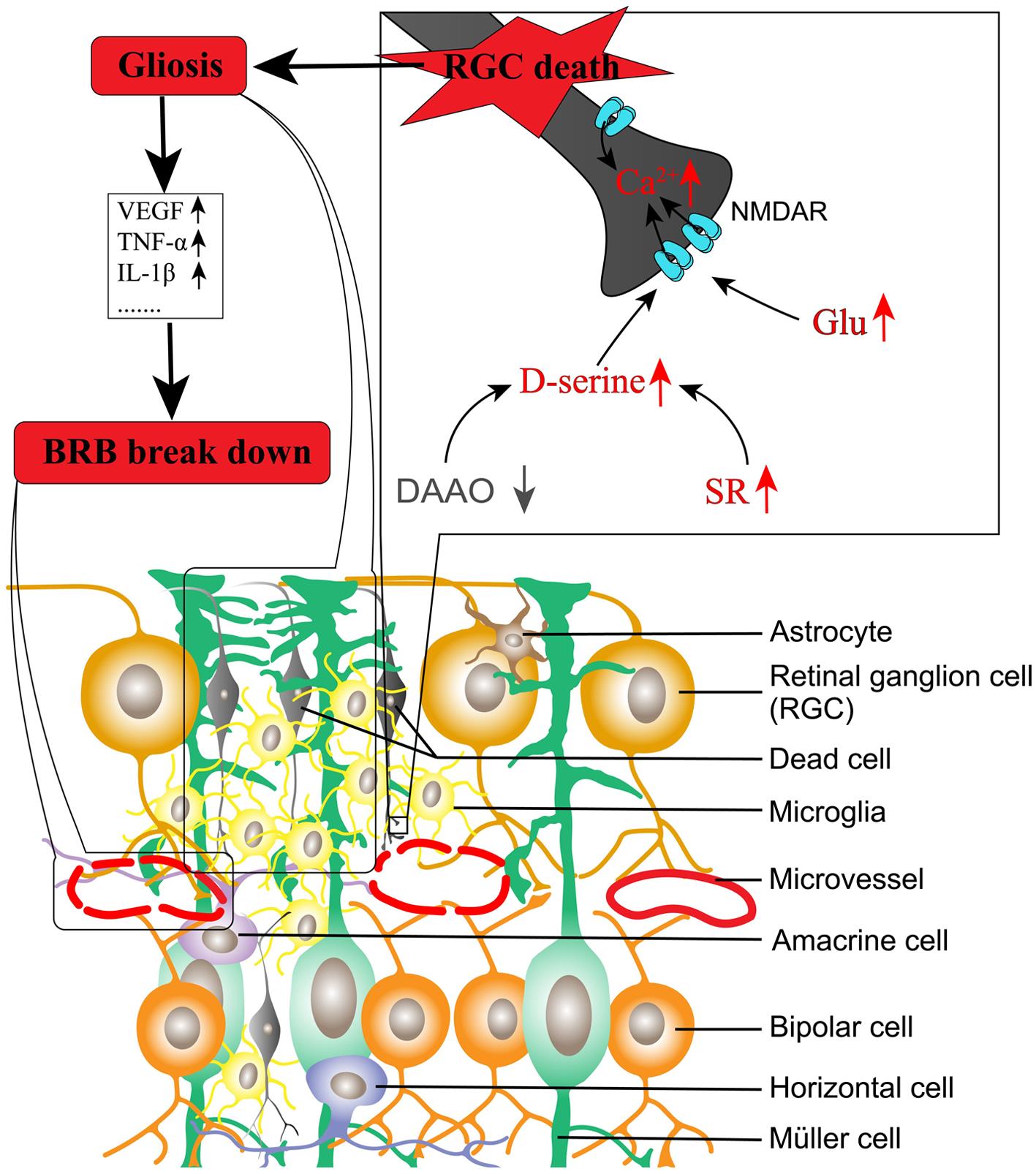
NMDAR activation is intimately associated with retinal neurodegeneration. In diabetes, the activity of glutamate transporter expressed on Müller glia is significantly decreased by oxidative stress and thus impairs glutamate metabolism.55,56 Diabetes also increases the expression of ionotropic glutamate receptor subunits in the human and rat retina.57,58 Meanwhile, an uncompetitive antagonist of NMDAR used for treating Alzheimer’s disease has been demonstrated to protect against retinal neurodegeneration and microvascular damage in diabetic rats.59 Considering that depletion of D-Ser largely attenuates NMDAR-mediated calcium influx and excitotoxicity,4,6 we explored the roles of SR and D-Ser in DR. Our results indicated the following: (1) SR is increased in the diabetic rat retina;60 (2) D-Ser is increased in the aqueous humor in diabetic rats and the aqueous and vitreous humor in diabetic humans;60,61 (3) loss of SR significantly reduces retinal neurodegeneration mediated by injected NMDA and in Ins2Akita mice, an animal model of type 1 diabetes;62,63 and (4) DAAO is decreased in the retina of diabetic rats, and overexpression of DAAO in the retina delivered by adeno-associated virus 8 prevents retinal neurovascular changes in diabetic rats.38 A study from another group also indicates that knockout of SR significantly reduces retinal neurodegeneration and loss of endothelial cells in retinal microvessels in diabetic mice.64 We propose that the imbalanced production of D-Ser coupled with extracellular glutamate in the diabetic retina contributes to DR. The model is depicted in Figure 4.
In models of diabetic retinopathy, SR is increased while DAAO is decreased in the retina compared to normal animals, leading to the increased production of D-serine. D-serine, coupled with increased glutamate in the extracellular compartment due to impaired glutamate transport, activates NMDAR, leading to RGC death. Consequently, glia including Müller cells, astrocytes, and microglial cells proliferate and release pro-inflammatory factors and pro-angiogenic factors, resulting in disruption of the blood-retinal barrier. BRB, blood-retinal barrier; DAAO, D-amino acid oxidase; Glu, glutamate; IL-1, interleukin-1; NMDAR, N-methyl-D-aspartate receptor; RGC, retinal ganglion cell; SR, serine racemase; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Similar to DR, glaucoma is a leading ocular disorder resulting in blindness worldwide. Long-term intraocular hypertension or the imbalance of the trans-lamina cribrosa pressure is associated with RGC degeneration and RGC axonopathy in glaucoma.65,66 Recently, studies from the same group have reported that D-Ser is significantly increased in the retina of glaucomatous animals and that depletion of D-Ser by DAAO prevents apoptosis of RGCs.67,68
Conclusion
Accumulated studies suggest that D-Ser is involved in the pathogenesis of DR and glaucoma. Therefore, regulation of D-Ser availability may provide a promising strategy to prevent or treat these disorders.
Abbreviations
- BRB:
blood-retinal barrier
- DAAO:
D-amino acid oxidase
- DR:
diabetic retinopathy
- D-Ser:
D-serine
- Glu:
glutamate
- IL-1:
interleukin-1
- L-Ser:
L-serine
- NMDAR:
N-methyl-D-aspartate receptor
- RGC:
retinal ganglion cell
- RPE:
retinal pigment epithelial
- SR:
serine racemase
- TNF:
tumor necrosis factor
- VEGF:
vascular endothelial growth factor
- ATP:
adenosine triphosphate
- CVD:
cardiovascular disease
- CM:
cardiomyopathy
- IS:
ischemic stroke
- mtDNA-CN:
mtDNA copy number
- mtDNA:
mitochondrial DNA
- MELAS:
mitochondrial neuro-gastro-intestinal encephalomyopathy
- MUTYH:
mutY DNA glycosylase
- nDNA:
nuclear DNA
- OGG1:
8-oxoguanine DNA glycosylase
- OS:
oxidative stress
- OXPHOS:
oxidative phosphorylation system
- POLG:
polymerase subunit gamma
- ROS:
reactive oxygen species
- TFAM:
mitochondrial transcription factor A
- 8oxoG:
7,8-dihydro-8-oxoguanine
Declarations
Acknowledgement
None.
Funding
The related studies have been supported by start-up funding (89210001) from Wenzhou Medical College, the National Natural Science Foundation of China (81371027), and an intramural grant: Integrated Project of the State Key Laboratory of the School of Optometry and Ophthalmology (#J02-20190204) to Dr. Shengzhou Wu.
Conflict of interest
The manuscript was submitted during Dr. Shengzhou Wu’s term from May 2023 to December 2024 serving as an editorial board member of Nature Cell and Science. The authors have no other conflicts of interest to declare.
Authors’ contributions
Critical revision of the manuscript for important intellectual content, administrative, technical, or material support (ZP). Study concept and design, drafting of the manuscript (WS).

 Author information
Author information